More Information
Submitted: May 19, 2025 | Approved: May 26, 2025 | Published: May 27, 2025
How to cite this article: Aljeboree AM, Mohamed BK, Abdulrazzak FH, Al-Mgheer TH, Alkaim AF, Himdan TA, et al. Fast identification for Evidences in Crime Scene with Macroscopic Properties and Portable Techniques. J Forensic Sci Res. 2025; 9(1): 087-091. Available from:
https://dx.doi.org/10.29328/journal.jfsr.1001084
DOI: 10.29328/journal.jfsr.1001084
Copyright License: © 2025 Aljeboree AM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Physical properties; Chemical properties; Nature of material; Evidence; Portable apparatuses
Fast identification for Evidences in Crime Scene with Macroscopic Properties and Portable Techniques
Aseel M Aljeboree1, Baraa Kasim Mohamed2, Firas H Abdulrazzak2*, Tariq H Al-Mgheer3, Ayad F Alkaim1, Takialdin A Himdan4 and Falah H Hussein5
1College of Science for Women, University of Babylon, Iraq
2Forensic Department, College of Science, University of Al-Karkh, Iraq
3College of Medicine, University of Babylon, Iraq
4Department of Pharmacy, Al-Esraa University College, Baghdad, Iraq
5College of Pharmacy, University of Babylon, Iraq
*Address for Correspondence: Firas H Abdulrazzak, Forensic Department, College of Science, University of Al-Karkh, Iraq, Email: [email protected]
In this work we are concerned with identifying visible evidence that can only be observed with the naked eye and is required for experiments by forensic scientists. The search details include many visible physical properties that could be used to explain many unknowns in the crime scene, which means critical truths for different cases. The details include appearance phenomena such as color, odor, shape, size, material type, and inferred properties based on physical appearance. The properties discussed in this work can be further analyzed using specific portable apparatuses that could give very important information about the structure and nature of the properties of evidence in a crime scene. We also deal in this work with general expertise, which forensic scientists should understand and could treat to make the process of identification and characterization in crime scenes be done more systematically in a short time.
As mentioned before in our previous work, the chemical compound can be used for identifying the identities of persons by modifying fingerprints [1]. On the other hand, chemical and microscopic identification for various organic and inorganic materials, simple or mixed in composition, is an essential component of identifying unknown materials and constitutes a fundamental unit of forensic science. This review discusses key chemical and microscopic properties that together constitute the comprehensive identification of materials in visible quantities, which commonly occur at crime scenes [2].
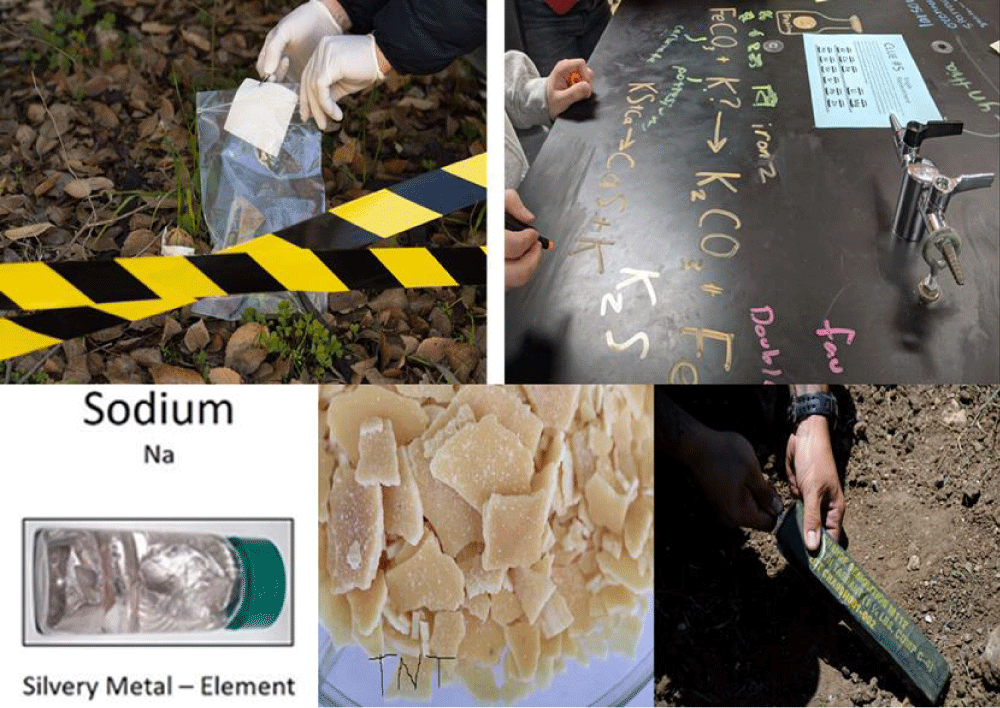
Identify the macroscopic properties that can be taken as a basis for knowing the apparent properties of matter, regardless of its types and physical states. To characterize a material with the best performance, you will need to consider the properties of the available materials. Figure 1 shows different evidence that required identifying the properties of observed characteristics and chemical properties that are responsible for determining the identities [3].
Figure 1: Overview of different types of evidence encountered at crime scenes.
At a crime scene, different samples in chemical and biological language are taken, which refer to evidence in forensic science fields. The first step that could be used by forensic workers is the visible evidence that could be seen firstly without any instruments and then followed by using instruments for more accuracy and to look for more evidence that could not be seen easily [4,5].
In this section, properties that could be identified include two parts: the first was physical and chemical properties, while the second part included smells and nature enhanced by portable apparatuses.
Physical and chemical properties
Physical properties: Part of physical properties describes how an object responds to mechanical forces. Hardness is an example of a mechanical property, the ability of a knife blade made from steel to drag a hard object, which is unchanged, while dragging a soft object with the blade across it, causing scratches. The response to force depends on the material's structure, shape, and size; however, a flexible object such as nylon can easily bend, while polyethylene needs more force to bend [6].
Elasticity refers to the ability of an object to return to its original shape and size after a force is removed. An object that breaks rather than bending is brittle. All of this behavior for different materials can be primarily dependent on identifying the identities of materials, but it is still required for more instruments to test physical properties, which could decide the name of unknown evidence [4,5].
A nylon windbreaker is strong, since pulling on it does not change its length. Sometimes the manner in which force is applied can affect an object's strength. Ceramics can bear a lot of weight, but will break if stretched or bent. Nylon withstands compression, pulling, and twisting [5].
Shining light on the surface of a sample reveals its color, texture, and reflectivity of evidence that could be found it in criminal scene such mirrors are colorless, smooth, and shiny. Applying heat to a sample reveals its ability to conduct heat, led to melting point, and boiling point respectively [5-7]. This section highlights the nature of properties and their relevance in material identification to make final decision for the identities of materials and that include intensive and extensive properties [5-8].
All of their properties, which should be known by forensic workers to make fast and direct identification of evidence that could be seen in crime scenes, are critical requirements to achieve fast and right prediction of the events [7].
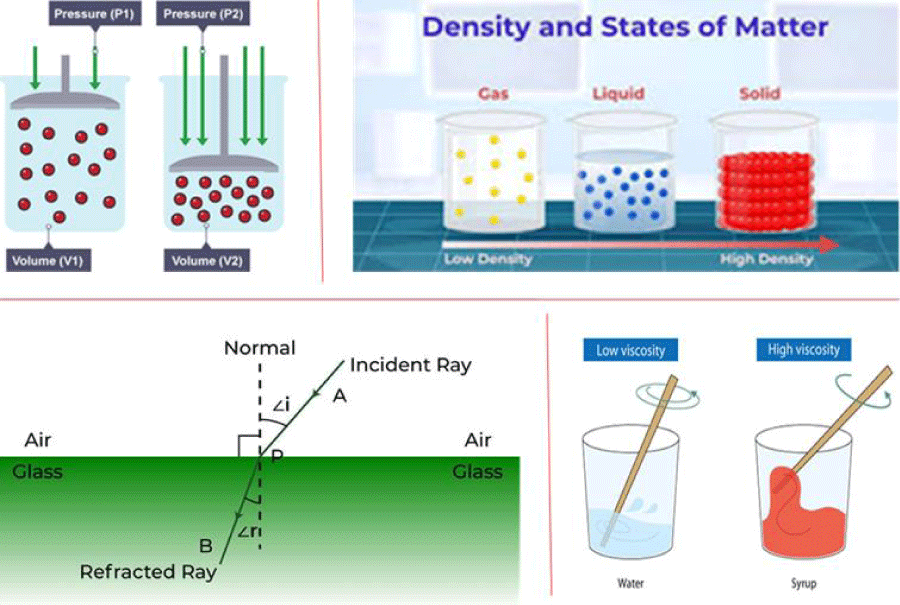
Some properties are independent of the amount of sample, such as melting point does not change if a sample is divided into small volumes, and mass and volume increase with the amount of sample being studied (Figure 2).
Figure 2: Illustration of physical properties including pressure, density, refractive index, and viscosity, right density, down, to the left refractive index, right viscosity.
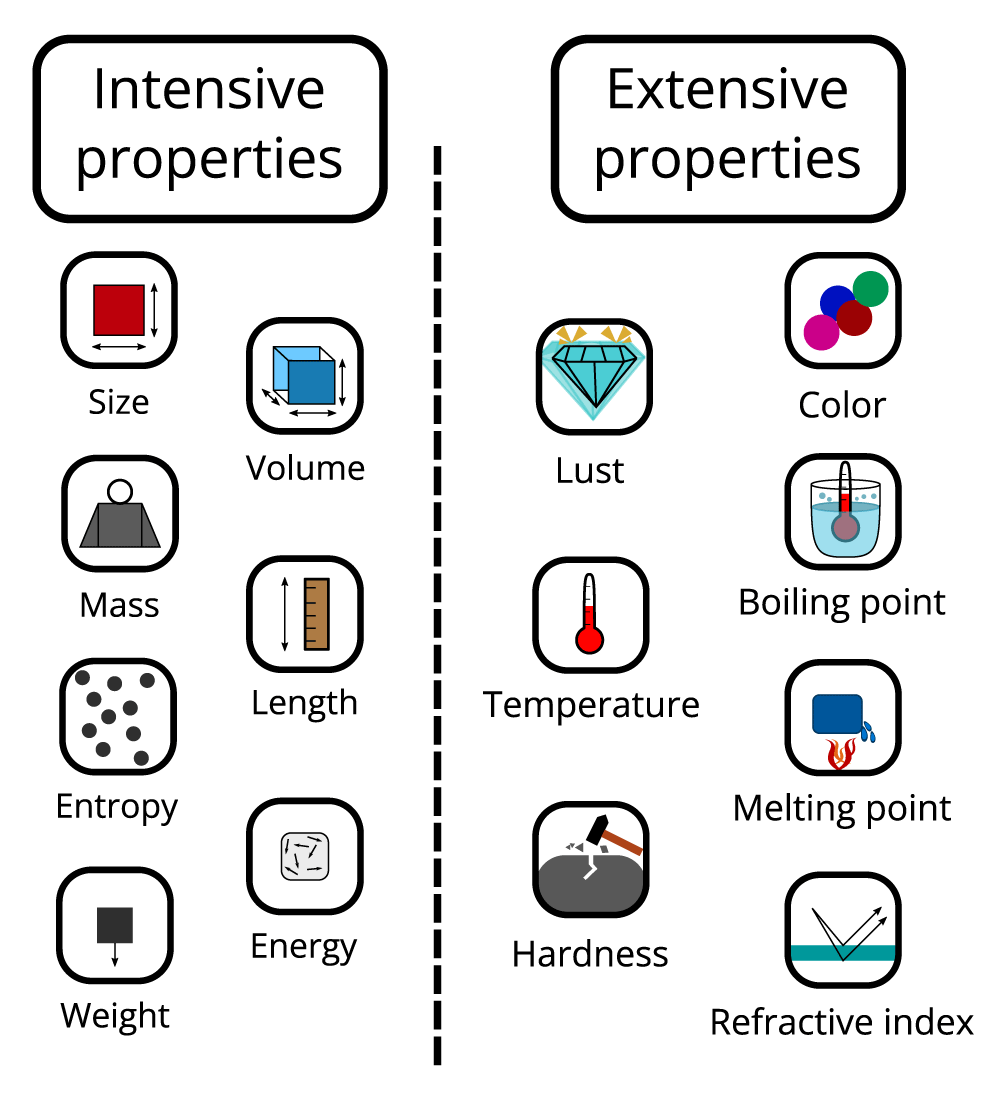
Intensive property: When the properties of matter that did not depend on the size or quantity of matter in any way were called intensive properties of matter, such as temperature, density, color, melting point, and boiling point, they would not change with a change in size or quantity of matter. As mentioned, this property can be used for preliminary identification, which is mostly used first in crime scenes before transferring the evidence to forensic labs.
Extensive property: All the property that was required for other properties to give a complete description, which means needed for size or the amount to give the identities of evidence in a crime scene, such as volume, energy, and mass. The extensive properties can be formed into intensive properties such as density when required for mass and volume (two are extensive); however, some properties are not measured directly but can be determined by combining measurements of other properties (Figure 3).
Figure 3: Skim for some intensive and expensive properties.
Chemical properties
These terms refer to very large and complicated terms that started with a reaction by nature and ended with many indirect reactions by people. This section outlines conditions commonly used to identify the identities of materials according to chemical properties. These descriptions include at first what is mentioned above about the reactivity of azide, perchlorate, or fulminate salts when crashing due to their ability to explode at room temperature by this action, not more [7-9]. A common example observed at crime scenes includes substances like sodium hydride, sodium hydrosulfite, and sodium metal with water, not more, leading to explosive reactions, spontaneous ignition, and flammability, respectively [9-12].
The disadvantage of chemical reactions at a crime scene includes not only risks to worker safety in forensic science which could be done by safety equipment but the damage which absolutely accord for the evidence in crime scene. This scientific opinion depends on simple role which is a chemical reaction involves a transformation of the sample into a different substance, and it may be difficult to reverse the process and prove existence many materials or it is at least complicated the looking for evidence. For example, flammable materials such as wood react with oxygen upon burning from the air and produces ashes and smoke it cannot be reversed in addition to loosening most if not all evidence [13-16].
Senses and portable apparatuses
Sense of smell and touch: Mostly forensic laboratory personnel often use smell to identify different gases, liquids, and solid evidence. Historically, such methods were used frequently, but have since been limited due to toxicity concerns in higher value because of toxic behavior for many compounds, when working with unknown substances [13-16] (Table 1).
Table 1: Physical properties (shape, color, smell, and existence) for identified common drags.
The other macroscopic property that can be used to distinguish between ionic and non-ionic compounds is “crunchiness.” When crushed with a spatula, most ionic compounds will feel crunchy, while non-ionic compounds will not. In this section, it should be a care crime scene (should not be tried with azide, perchlorate, or fulminate salts because these tend to be explosive) [13-17].
In this part, it is important to be concerned with two complementary terms, which are state functions and path functions. In the forensic sciences the first terms which is state functions refers to evidence that could be depend as it is without dealing with the history of evidence. The second terms path functions include all the evidence that found in crime scene which should trace its history, starting from the location where it was found because it needed for more information to give clear images for the event [14-20]. According to physical chemistry, the properties considered state functions depend only on initial and final conditions in the crime scene to be understood from forensic science, while the other required more steps to be completely understood.
Maybe the best example of a state function and path function and relation with evidence is the sublimation process when the materials exist in a solid state and convert to gases or vapor state. This physical behavior implies that some materials may temporarily exist at a crime scene and disappears without any fingerprint for anyone at room temperature or without left observe material which refers to it. The first example: dry ice, which is a frozen form of carbon dioxide, which directly changes its phase from a solid-state to a gaseous state when exposed to air. The other example is represented by Naphthalene, which is an organic compound with repulsive intermolecular forces due to non-polar behavior [15-22]. These properties are important information when treated with critical conditions at a crime scene if there is the ability to use physical properties of different materials to cause abnormalities in normal conditions [15-23].
Apparatuses for measuring properties: The information mentioned above concerns using experiments for a scantest in the forensic field to observe, locate, identify, and at least identify primary identities by macroscopic properties before going to the lab [15-18]. Portable apparatuses serve as critical field tools for forensic scientists that are able to give much information about the evidence in a few seconds or in a few minutes. Table 2 includes several portable types, which are density meter, viscometer, refractometer, UV-visible spectroscopy, Raman spectroscopy, and IR spectroscopy. All of these techniques and others [19-22] are able to enhance the primary identification for evidence with good confidence. The most important advantage for these apparatuses is represented by (1) their ability to be used in different conditions without initial preparation or complicated analysis. (2) The other benefit was the high speed for getting analysis with good agreements. (3) High safety without remaining or producing any waste. (4) Most of these apparatuses provided with memory capacity capable of storing large amounts of test data without requiring additional components.
Table 2: Skim for portable apparatuses used in crime scene for identify many properties.
Assessment of evidence identification
The process of evaluating identification results requires specific metrics which is the limiting concentration (L. C.), identification (L. I.) and proportions (L. P.) respectively. The first refers to the lowest concentration of substance, which always produces a positive test. The second (L. I.) is the smallest absolute quantity that always gives a positive test, while (L. P.) are the smallest mass ratios that still always permit getting a positive test [22,23]. The value of L.C. absolutely depends upon the absolute size of the sample or the volume of solution taken for the test and may be improved by reducing the scale of work. According to that, L.P. is less favorable than L.I. or L.C., which required isolating the properties to be more detected by method [24-26].
Limiting concentrations, limits of identification, and limiting proportions cannot be determined with high accuracy since the conditions that always give a positive test and those that always lead to a negative result have no sharp borderline. They are separated by a range of conditions, which produces uncertainty as to the outcome: some trials give positive and some' negative results. This implies that the conditions considered to 'always' yield a positive result must be established by a sufficient number of experiments. One of the aims of this work is to distinguish between trace evidence which could be seen directly without any instruments and trace evidence that was found in low quantities which required using specific instruments to identify it [27,28]. Finally, we are concerned with the first look at a crime scene and looking for evidence before going to the lab, which is an important and critical requirement to make the analysis in the forensic lab ideal and complete to uncover the truth through scientific and systematic legal procedures.
Through this work, the focus was on the specific aspect of uncovering evidence at the crime scene. This relied on the forensic scientist's ability to recover evidence, describe it, and make an initial decision. As noted, the decision depends on the forensic expert’s experience and available preliminary evidence accumulated by workers in the forensic field. This information enables preliminary case assessments at crime scenes with reasonable confidence. In this section, it should refer to the importance of macroscopic properties that could be noted and characterized to transform it from an unknown to a known state, which is critical in explaining many discrepancies at crime scenes under varying conditions. The supplement enhances the accuracy and confidence of the initial examination and can be done using portable apparatuses that are able to show necessary information in a short time. In addition to this, the results can be assessed with high confidence.
- Aljeboree AM, Radhi IM, AbdulLatif AI, Abdulrazzak FH, Abbas AM, Alkaim AF, Himdan TA, Hussein FH. Review: Synthesis of Chemical Materials for Fingerprint Detection. Int J Innov Sci Res Technol. 2024;9:2720-6. Available from: http://dx.doi.org/10.38124/ijisrt/IJISRT24JUL1675
- Rendle DF. Advances in chemistry applied to forensic science. Chem Soc Rev. 2005;34(12):1021-30. Available from: http://dx.doi.org/10.1039/b415890n
- Milman BL. Principles of Identification. In: Milman BL, editor. Chemical Identification and its Quality Assurance. Berlin, Heidelberg: Springer; 2011. Available from: https://doi.org/10.1007/978-3-642-15361-7_1
- Pratap CE. Recent Trends in Crime Scene Investigation: Inclusion of Forensic Science under New Criminal Law. ISAR J Sci Technol. 2025;3(1):1-5. Available from: https://article.isarpublisher.com/viewArticle/Recent-Trends-in-Crime-Scene-Investigation-Inclusion-of-Forensic-Science-under-New-Criminal-Law
- Jang M. Exploring the quantity and type of evidence collected during criminal investigations in South Korea. Forensic Sci Int Synergy. 2024;9:100544. Available from: https://doi.org/10.1016/j.fsisyn.2024.100544
- Hawley-Morelos H, Cortes-Perez OI. Crime Scene and Feminicide: Crime Scene Investigation and Processing Standards. In: Cortes-Perez OI, editor. Forensic Victimology and Femi(ni)cide. Cham: Springer; 2024. Available from: https://doi.org/10.1007/978-3-031-72512-8_6
- Kleypas DA, Badiye A. Evidence Collection. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441852/
- Liang C. Understanding Plastic Deformation: Principles, Mechanisms and Applications. Adv Mater Sci Res. 2024;7(6):229-30. Available from: https://www.openaccessjournals.com/articles/understanding-plastic-deformation-principles-mechanisms-and-applications-18214.html
- Caire G, Sano P. EPISD Forensics Team. 2nd ed. 2016. Available from: https://www.newcastle.k12.ok.us/Downloads/EPISD-Forensic-Science_b_v200_hkb_s1.pdf
- Trejos T, Koch S, Mehltretter A. Scientific foundations and current state of trace evidence—A review. Forensic Chem. 2020;18:100223. Available from: https://doi.org/10.1016/j.forc.2020.100223
- Bräse S, Gil C, Knepper K, Zimmermann V. Organic Azides: An Exploding Diversity of a Unique Class of Compounds. Angew Chem Int Ed Engl. 2005;44:5188-240. Available from: https://doi.org/10.1002/anie.200400657
- Belle WV. Fatal Chemical Decomposition Reaction and Explosion at Optima Belle LLC. U.S. Chemical Safety and Hazard Investigation Board; 2023. Available from: https://www.csb.gov/assets/1/6/optima_report_for_publication.pdf
- Furstenberg R, Nguyen V, Fischer T, Abrishami T, Papantonakis M, Kendziora C, et al. Advances in sublimation studies for particles of explosives. In: CBRNE Sensing, 2015. Available from: http://dx.doi.org/10.1117/12.2177390
- Breshike CJ, Kendziora CA, Furstenberg R, et al. Infrared backscatter imaging spectroscopy of trace analytes at standoff. J Appl Phys. 2019;125(10):104901. Available from: https://doi.org/10.1063/1.5079622
- Laska PR. Safety at the Crime Scene. Law Enforc Technol. 1997;24(8):25-8. Available from: https://www.ojp.gov/ncjrs/virtual-library/abstracts/safety-crime-scene
- Lease N, Spielvogel KD, Cawkwell MJ, Manner VW. Synthesis and investigation into explosive sensitivity for a series of new picramide explosives. J Energ Mater. 2024:1–21. Available from: https://doi.org/10.1080/07370652.2024.2446882
- Stahl H. Forensic Analysis of Lethal Toxin in Criminal Cases. J Forensic Toxicol Pharmacol. 2024;13(4). Available from: https://www.scitechnol.com/peer-review/forensic-analysis-of-lethal-toxins-in-criminal-cases-WvK6.php?article_id=27338
- Pautrat M, Barbier E, Lebeau JP. Identifying available substance use disorder screening tests feasible for use in primary care: A systematic review. Prev Med Rep. 2024;38:102610. Available from: https://doi.org/10.1016/j.pmedr.2024.102610
- van Asten A. Analytical chemistry in the forensic laboratory. In: Chemical Analysis for Forensic Evidence. Elsevier; 2023. p. 25–64. Available from: https://doi.org/10.1016/B978-0-12-820715-4.00003-1
- Franjić S. Forensic Chemistry is an Important Part of any Forensic Investigation. Mathews J Foren. 2022;3(1):10. Available from: https://www.mathewsopenaccess.com/full-text/forensic-chemistry-is-an-important-part-of-any-forensic-investigation
- de Araujo WR, Cardoso TMG, da Rocha RG, Santana MHP, Muñoz RAA, Richter EM, et al. Portable analytical platforms for forensic chemistry: A review. Anal Chim Acta. 2018;1034:1-21. Available from: https://doi.org/10.1016/j.aca.2018.06.014
- Trombka JI, Schweitzer J, Selavka C, Dale M, Gahn N, Floyd S, et al. Crime scene investigations using portable, non-destructive space exploration technology. Forensic Sci Int. 2002;129(1):1-9. Available from: https://doi.org/10.1016/S0379-0738(02)00079-8
- Zhang J, Li F, Yu L, Wang Y, Wang K, Chang C, et al. Research on the explosive characteristics and suppression mechanisms of gas generation during thermal runaway of batteries in a charged state. Chem Eng J. 2025;505:159699. Available from: https://doi.org/10.1016/j.cej.2025.159699
- Lennox-Steele A, Nisbet A. A forensic examination of several mobile device Faraday bags & materials to test their effectiveness. In: Valli C, editor. Proc 14th Australian Digital Forensics Conference. 2016;34–41. Available from: https://ro.ecu.edu.au/adf/165/
- Committee to Review the IRIS Process; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council. Review of EPA's Integrated Risk Information System (IRIS) Process. Washington (DC): National Academies Press (US); 2014. Chapter 4, Evidence Identification. Available from: https://pubmed.ncbi.nlm.nih.gov/25101400/
- Deffenbacher KA. Review of Identification Evidence: A Psychological Evaluation. In: Shepherd JW, Ellis HD, Davies GM, editors. Am J Psychol. 1983;96(4):591–5. Available from: https://www.jstor.org/stable/1422589
- Zughaibi TA, Assiri Z, Mirza A, Alharbi H, Alzahrani AE, Alahmadi SA, et al. A Quantitative and Comparative Study of Heroin-Related Metabolites in Different Postmortem Fluids and Tissues. Toxics. 2025;13(3):229. Available from: https://doi.org/10.3390/toxics13030229