More Information
Submitted: April 15, 2025 | Approved: April 30, 2025 | Published: May 01, 2025
How to cite this article: Swargiary SD, Pandey P. Touch DNA Recovery from Non-porous Surfaces. J Forensic Sci Res. 2025; 9(1): 041-049. Available from:
https://dx.doi.org/10.29328/journal.jfsr.1001079
DOI: 10.29328/journal.jfsr.1001079
Copyright License: © 2025 Swargiary SD, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Touch DNA Recovery from Non-porous Surfaces
Silpi Das Swargiary* and Preeti Pandey
Lovely Professional University, India
*Address for Correspondence: Silpi Das Swargiary, Lovely Professional University, India, Email: [email protected]
Touch DNA, the minute quantities of DNA deposited through skin contact, has become a valuable tool in forensic investigations. However, the recovery of touch DNA from non-porous surfaces remains a challenging task, requiring optimized collection and extraction techniques to maximize DNA yield, because non-porous surfaces have smooth, non-absorbing material properties. This review explores various non-porous surfaces such as glass, plastic, and metal, analyzing their impact on DNA recovery efficiency. Different collection methods, including swabbing, tape lifting, scrubbing, and vacuum collection methods, are evaluated to determine their effectiveness in retrieving minute amounts of DNA from these surfaces.
Through a comparative analysis of existing studies, this paper identifies which collection methods work best for different non-porous surfaces and why choosing the right technique matters. Factors such as surface type, environmental conditions, and collection technique performed, time duration, and so on can affect DNA recovery, making it crucial to use the most effective approach. This review also emphasizes the need for standardized protocols to ensure consistent and reliable results in forensic investigations. Having clear guidelines can reduce errors, improve DNA analysis, and make touch DNA analysis more reliable in forensic investigations. By focusing on these aspects, this study aims to contribute to the ongoing efforts in refining touch DNA recovery strategies.
Touch DNA is important in forensic investigations, is the genetic material that is left on surfaces by casual contact, such as perspiration or skin cells. Because non-porous materials—such as glass, metal, and plastic—cannot absorb biological material like porous surfaces do, they pose special difficulties for DNA recovery. Touch DNA from non-porous surfaces can be essential in linking suspects to crime scenes in spite of these difficulties, especially in situations when additional evidence is missing [1-5].
Crime scenes frequently feature non-porous materials like glass, metal, and plastic, which may hold onto contact DNA for a long time. This allows for the recovery of traces of DNA even after a delay Budowle, et al. A suspect can be connected to a crime by skin cells left on items like firearms or doorknobs [6]. But since the DNA is weakly bonded and readily removed or deteriorated by environmental conditions, retrieving it from these surfaces is difficult [7]. Recovery and analysis are further complicated by the tiny amount of DNA that is left behind [8].
Importance of DNA recovery from non-porous surfaces
Numerous objects, such as tools, weapons, door handles, and personal possessions, can contain touch DNA. Using touch DNA analysis, forensic experts can help prove the legal evidentiary connection between the suspect and the crime scene, offer further proof for identification, or disassociate innocent people from suspicion. Therefore, it is crucial to remember that touch DNA analysis has limitations of its own. Environmental factors, surface type, contact type, duration and intensity of contact, and other factors can all affect the quality and amount of touch DNA. Because touch DNA can be readily tainted by other DNA sources, appropriate methods of collection and preservation are essential to guaranteeing reliable results [9].
The science of DNA
DNA (deoxyribonucleic acid) is a linear polynucleotide made up of four different kinds of monomeric nucleotides. A phosphate group, a nitrogenous base, and a deoxyribose make up each nucleotide. The four building blocks of DNA are thymine (T), guanine (G), cytosine (C), and adenine (A). A base's nitrogen has the deoxyribose bonded to it. Attached to the deoxyribose is the phosphate group. Phosphodiester bonds bind the individual nucleotides in a polynucleotide together [10].
Tables 1-5
| Table 1 | ||
| Author | Year | Focus/Contribution |
| Sinukaban, et al. | 2024 | Studied DNA quantification on glass, plastic, and ceramic. Found poor extraction quality using Chelex, highlighting need for improved techniques. |
| Kaesler, Kirkbride & Linacre | 2023 | Tracked DNA persistence on fabric, steel, and rubber across 9 months; indoor vs. outdoor conditions impacted DNA survival on non-porous surfaces [11,12]. |
| Zhang, et al. | 2022 | Comparative review of swabbing, tape lifting, vacuuming; concluded no universal best method—surface and condition-dependent. |
| Kaur, et al. | 2022 | Reviewed entire touch DNA workflow including analysis, emphasizing importance of optimizing every step, not just collection. |
| Alketbi & Goodwin | 2021 | Compared nylon vs cotton swabs; nylon with moist solution yielded higher DNA on non-porous surfaces [13-16]. |
| Table 2: Challenges and limiting factors. | ||
| Author(s) | Year | Focus/Contribution |
| Sinha, Yadav & Bumbrah [17]. | 2021 | Discussed limitations of touch DNA like contamination risks and low quantity; stressed careful sample handling. |
| Hedman, et al. | 2020 | Showed single wet swab outperforms second dry in double-swab method; emphasized technician handling over method alone [18,19]. |
| Bonsu, et al. | 2020 | Reviewed DNA recovery from metal; identified metal corrosion and surface characteristics as limiting factors. |
| Nimbkar & Bhatt | 2022 | Suggested combining vacuum collection with pre-wetting for effective sampling from large, irregular non-porous surfaces. |
| Table 3: Advanced methods and technological solutions. | ||
| Author(s) | Year | Focus/Contribution |
| Tozzo, et al. | 2022 | Provided systematic review of collection techniques, found moist swabbing and FTA cards promising for trace collection [20]. |
| Alketbi [13] | 2023 | Reviewed direct amplification protocols for touch DNA recovery; emphasized reducing sample loss during transfer. |
| De Alcaraz-Fossoul et al. | 2023 | Studied DNA recovery after submersion in spring water; found material type affected persistence on non-porous surfaces [21]. |
| Comte, et al. | 2019 | Evaluated four swab types, finding flocked swabs superior for collecting trace DNA. (Referenced in newer studies post-2020) [22] |
| Li, et al. | 2017 | (Used in 2024 study as foundation) Tested vacuuming on ceramic, plastic, and glass; efficient for large-area sampling [23]. |
| Table 4: Foundational studies and their ongoing relevance. | ||
| Author(s) | Year | Focus/Contribution |
| Martin & Cotter | 2014 | (Cited in 2021–2024 studies) Found FTA cards effective for long-term storage and high integrity preservation |
| Dong, et al. | 2017 | Compared preprocessing and storage time impacts; newer studies validated these protocols for forensic contexts. |
| Replogle & Andrews | 2020 | Introduced electrostatic lifting for delicate surfaces; shown effective for minimal contact recovery [24]. |
| Ballantyne & Van Oorschot [1] | 2015 | Early baseline on DNA transfer properties still referenced in 2021–2024 papers. |
| Krane & Lund [8] | 2016 | Discussed sample misinterpretation in low copy DNA—foundational in most recent analytical approaches. |
| Gray & Passmore [7] | 2018 | Environmental degradation insights applied in modern touch DNA studies for protocol development [25]]. |
| Butler JM. [26] | 2010–2012 | His DNA typing and extraction works are still foundational in extraction methods cited in new reviews. |
| Sood & Gautam | 2021 | Emphasized Locard’s principle in modern crime scene applications for touch DNA. |
| Pang & Cheung | 2007 | First introduced double swab technique, still being revalidated in 2020+ studies. |
| Forsberg, et al. | 2016 | High-throughput DNA tape extraction protocols, used as a benchmark in recent vacuum vs. tape discussions. |
| Table 5: Recent applications and case studies. | ||
| Author(s) | Year | Focus/Contribution |
| Williams, et al. | 2024 | Linked touch DNA to cold case resolution; highlighted potential of new sequencing methods. |
| Burrill, Daniel & Frascione | 2019 | Discussed multiple DNA sources like cell-free DNA; many 2020s papers build upon this [27]. |
| Tang et al. | 2020 | Quantified DNA loss from synthetic fingerprints; findings impacted recovery strategy design. |
| Thomasma & Foran | 2013 | Showed that swab solution choice alters yield—a key variable in modern recovery research. |
| Wickenheiser | 2002 | Pioneering touch DNA theory—most modern papers include his transfer model in theoretical sections. |
| Van Oorschot, et al. | 2010 | Introduced concepts around secondary transfer; cited heavily in 2020s as baseline. |
Research gap
As much as the area of touch DNA recovery from non-porous surfaces has advanced, there is still work left to be done on the following issues which hinder the reliability and reproducibility of forensic evaluation:
Lack of universal collection protocols
There is no universal protocol for the collection as a number of processes such as swabbing, tape lifting, and vacuuming have been provided [13,28]. Most methods, including those evaluated in collection studies, are still situational and greatly reliant on the skill level of the practitioner. These approaches result in variable DNA retrieval outcomes depending on context.
Surface-specific limitations
De Alcaraz-Fossoul, et al. and Sinukaban, et al. observed that the physical surface of a material and its chemical constituents together with environmental factors greatly affect the persistence of DNA on the surface. Comparison studies on non-porous surfaces, particularly contemporary materials such as polymer coated glass and brushed alloys, are scarce [29,30].
Limited real-world scenario validation
Most of the research conducted to date has been done in the laboratory. There is a lack of forensic simulation studies that evaluate method performance with respect to time for a variety of conditions such as high humidity, UV light, mixed deposition of DNA which are commonly encountered at crime scenes Kaesler, et al. 2023; Bonsu, et al.
Advanced technologies that have received little attention
Both vacuum-based and electrostatic collection methods Replogle & Andrews, 2020; Nimbkar & Bhatt, 2022 demonstrate potential; however, their implementation is lukewarm due to a lack of practical validation in the field and financial constraints. For operational forensic purposes, refinement of forensic techniques is still a work in progress.
Extraction efficiency vs. DNA integrity
As previously mentioned, the efficiency of DNA extraction using new techniques remains debatable [26,31]. Critical interactions between collection techniques and DNA integrity post-collection require further study, particularly in cases of limited or degraded samples.
Touch DNA from digital devices
Actions taken in relation to digital devices in the context of criminal proceedings have been well documented; however, there is very little research regarding the efficiency of recovering DNA from electronic surfaces such as screens and keyboards, which are deeply related to actual crime scenes [32].
Environmental impact studies are scarce
Research conducted on the degradation of DNA has been widely accepted [7], but few have scrutinized the effects of the combination of one or more of these factors—temperature, humidity, and UV radiation—on non-porous surfaces of DNA in bulk quantity and quality.
Sinha, et al. [17] and Gill & Solsberg [6] show that the inconsistency in shedder status, contact pressure, and handling procedures greatly affect the forensic DNA transfer and recovery processes. Nonetheless, there remains a struggle to measure biological variables and incorporate them into collection protocol optimization [33].
Definition and source
The term "touch DNA" refers to DNA that is transferred from a person to an object through direct contact. This type of evidence has also been referred to as "contact DNA," "trace DNA," or "transfer DNA" in the literature [31]. In the past, scientists thought touch DNA only came from skin cells shed externally, but recent studies show it can come from different places like saliva, nasal fluids, or body parts touching the hands. Sweat may also contain DNA. Even though hand skin cells don’t shed a lot, they still add to touch DNA if they’re transferred from other parts of the body. Thus, touch DNA may originate from various body sources beyond hand cells. Experiments show skin surfaces contain a bit of DNA, possibly from the outer layer of the skin or sweat Sessa, et al. DNA deposited by touch originated from shed keratinocytes, certain research findings provide a broader view by identifying a number of sources, including complete or partial skin cells, nucleated epithelial cells from other bodily fluids or parts that come into contact with the hands (such as sweat, sebum, or saliva), or cell-free DNA that is either endogenous or transferred onto the contact region from the previous fluids [27].
DNA evidence can be obtained from biological materials such as skin cells, blood, and hair Williams, et al. DNA testing technology has the potential to solve prior crimes that were committed before it was developed. Touch DNA, which refers to DNA left behind from Casual contact with objects has become an essential tool in forensic investigations Williams, et al.
Principle of mutual exchange (Locard's principle)
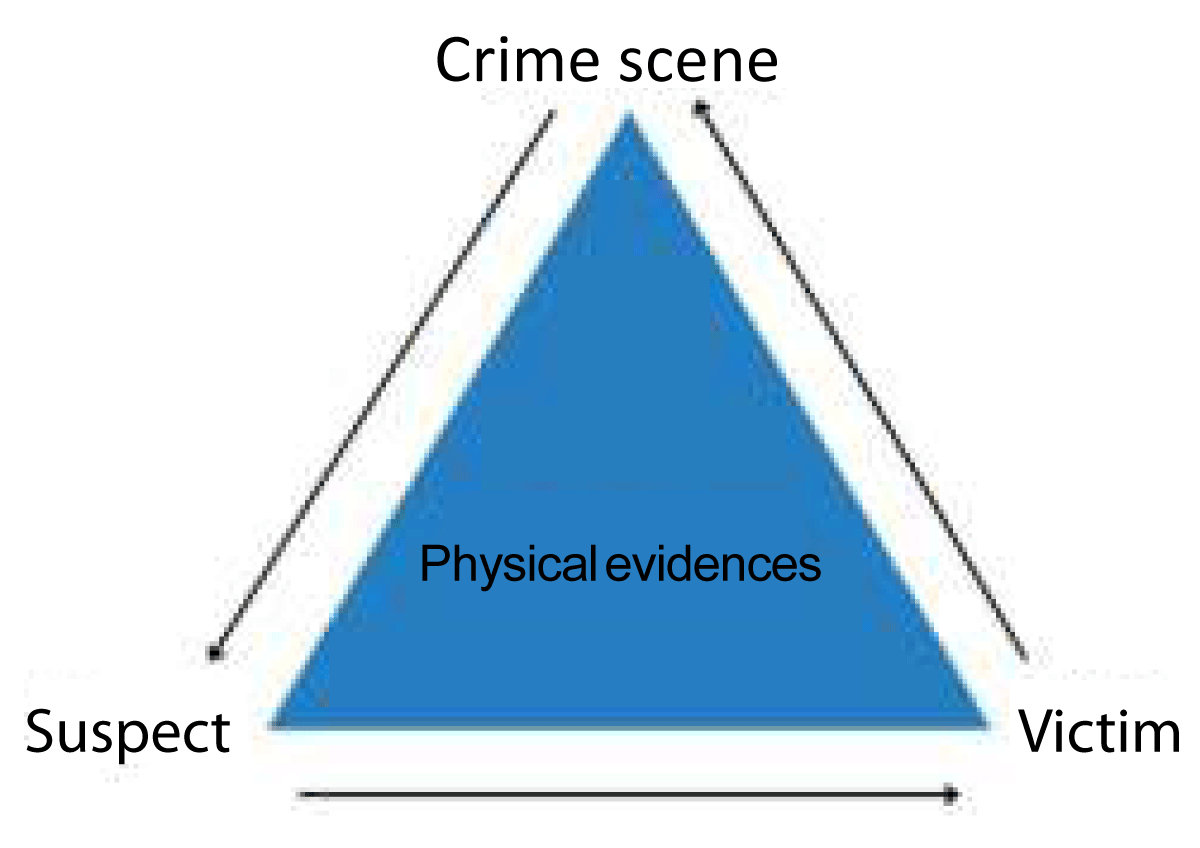
Unquestionably, Edmond Locard is most known for developing Locard's Principle of the theory of mutual exchange, which pertains to the transfer of trace evidence between things that they encounter. Another way to express the idea is as follows: "a trace is left by every contact" (e- PG Pathshala) (Figure 1).
Figure 1: Physical evidences helps in linking the case.
Edmond Locard, a French scientist, was the first to articulate this idea. It says that there will always be a reciprocal exchange when two surfaces come into contact of materials over the boundary of contact, that is, when a criminal or his criminal tools comes into contact with the sufferer or nearby objects, they will constantly exchange traces of each other. This principle states that it is nearly impossible for a person to commit a crime. This theory states that it is nearly hard for a criminal to carry out an act without leaving behind evidence and taking it with them. Similarly, the criminal or his tools also detect traces from the same contact. Scientific crime investigation is based on the trace evidence left on the crime scene and on the accused, which, if correctly examined, can establish a conclusive link between the suspect and the victim or determine their presence with the crime scene Sood A & Gautam A.
Sources of touch DNA
Epithelial cells: The skin is the body’s largest organ, making up 7% - 15% of its total weight. These cells form a layer called the stratified epithelium that covers the body’s outer surface. The outer layer of the skin, the epidermis, continuously renews itself as basal cells divide and mature slowly. Touch DNA primarily originates from the exfoliation of epithelial cells from the outermost layer of the skin (epidermis).
Sweat: Sweat, a complex fluid secreted by eccrine and apocrine glands, may contribute to touch DNA. While sweat contains epithelial cells, its DNA concentration is generally lower than that of skin cells.
Saliva: Saliva contains buccal epithelial cells from the oral mucosa, as well as DNA from leukocytes present in the saliva.
Mucus: Transfer of mucus to surfaces can occur through activities such as coughing, sneezing, or touching the face.
Other bodily fluids: Various bodily fluids, including semen, vaginal fluid, and urine, may contain cellular material and DNA. Transfer of these fluids to surfaces through contact can contribute to touch DNA deposition.
Factors affecting touch DNA
Numerous factors affect the transfer of DNA and its subsequent recovery, which in turn affects the recovery of touch DNA from crime scenes. These variables include the surface type, the mode of contact, the environmental factors, and the DNA collection methods.
Surface type: The DNA's ability to be recovered is greatly influenced by the type of surface it is deposited on. Unlike porous surfaces, which may absorb and trap DNA, non-porous surfaces, such as glass, metal, and plastic, usually keep DNA on the surface, making it easier to collect. However, if the DNA is damaged or deposited weakly, non-porous surfaces may still provide problems [1].
Manner of contact: The amount of DNA that is transferred can be influenced by how an individual contact with a surface .Only small amount of DNA remain after a quick or light touch and these can be challenging to retrieve. On the other hand, additional DNA maybe deposited by prolonged or strong contact, which would increase the chance of recovery [6,34].
Environmental conditions: The amount of DNA that stays on a surface can be affected by Environmental factors such as temperature, humidity, and UV radiation exposure. In severe adverse conditions, DNA might break down more quickly, lowering the amount and quality of the sample that can be analyzed. For instance, excessive heat and humidity might hasten DNA deterioration, making recovery more difficult [7].
Method of collection: The amount of DNA retrieved greatly depends on the method employed to collect it. Techniques like scraping, tape lifting, and moist swabbing can all affect how much DNA is removed from a surface. The type of swabbing solution, the pressure applied during collection, and the duration all have an impact on the effectiveness of DNA recovery [8]. Using the best collection methods is essential to maximizing DNA yield, especially when the DNA is present in trace amounts.
Types of surfaces
Surface properties have a big impact on the collection and extraction procedure for recovering touch DNA. The two basic categories of surfaces: porous and non-porous: each have unique effects on the effectiveness of DNA recovery [35](Figure 2).
Figure 2: Types of surfaces.
Porous surfaces
Porous surfaces have small pores that absorb liquids and particles, including skin cells, to be absorbed into the material.
Some common examples are:
| Porous Material | Examples |
| Paper | Found in documents, envelopes, and packaging |
| Fabric/Cloth | Clothing and carpeting |
| Wood | Furniture, flooring, and various household objects |
| Cardboard | Used in boxes, storage containers, and product packaging |
On porous surfaces, touch DNA tends to be absorbed into the fibers or structure, which can make it challenging to collect. Traditional swabbing may yield limited DNA due to deeper absorption, so alternative methods, such as cutting or scraping parts of the material, are sometimes employed. The advantage of porous materials, however, is that they offer some protection to DNA, preserving it from environmental degradation factors like UV light or heat. This preservation can sometimes lead to higher DNA yield despite collection challenges [36-40].
Non-porous surfaces (e.g. glass, metal, plastic, ceramic, etc.) [41]
Materials that don't absorb liquids or biological debris are referred to as non-porous surfaces. Because these surfaces can retain touch DNA left behind by casual contact, they are frequently used in forensic investigations [42-45].
Due to the lack of absorption, DNA on non-porous surfaces is typically collected using a moistened or dry swab, which can lift cells directly from the surface. However, DNA on non-porous materials is more exposed to environmental factors, which can lead to degradation or contamination. For instance, exposure to moisture, UV light, or high temperatures can quickly degrade touch DNA, reducing its quality and quantity [46-49].
Common types of non-porous surfaces are listed in Table 6.
| Table 6: Non porous materials and example [13,97,98]. | |
| Non-Porous Material | Examples |
| Glass | Found in windows, mirrors, bottles, and other glassware [99] |
| Plastic | Used in electronics, containers, bags, and household items |
| Metal | Common in door handles, tools, and various metal surfaces [100] |
| Ceramic | Present in tiles, plates, and pottery [83] |
Characteristics of non-porous surfaces that affect DNA recovery
According to De Alcaraz-Fossoul, et al. these characteristics include the surface's chemical makeup, texture, and smoothness as well as the type of contact that occurs between the person and the surface.
Smoothness: Although they don't trap DNA like porous materials do, smooth, non-porous surfaces like glass or polished metal have a tendency to hold onto it. In contrast to porous surfaces, these surfaces might facilitate DNA recovery, but they can also cause DNA to be deposited in a thin, dispersed layer that makes efficient collection challenging [50,51].
Surface roughness: A non-porous surface's roughness can influence how readily DNA sticks to it. Compared to flat surfaces, rougher materials—like textured plastic or brushed metal— may be better at retaining DNA. Because of the surface flaws, textured materials' increased surface area can facilitate DNA transfer more effectively, but it can also make recovery more challenging [52].
Chemical composition: A non-porous surface's makeup can also affect how well DNA is retained. Certain metals, for instance, have the ability to interact with DNA and change its integrity or cause deterioration. Similar to this, some metal or plastic coatings or finishes can hinder DNA recovery by either making it more difficult for DNA to stick to the surface or by preventing ideal swabbing [53].
Environmental exposure: Heat, moisture, or exposure to sunlight can degrade DNA on non- porous surfaces. Although the DNA may not be absorbed by the surface, environmental conditions can affect how long DNA can be detected and recovered [54] (Table 7).
| Table 7: Comparative analysis of collection methods for touch DNA on non-porous surfaces. | |||||
| Collection Method | Description | Optimal Surface Type | Effectiveness | Optimized Use | Author(s) |
| Dry Swabbing | Using a dry cotton or foam swab to collect surface DNA | Smooth, non-porous (glass, plastic) | Low-Moderate | Low cost, easy to use, but may miss DNA | Ballantyne & Van Oorschot [1], Tozzo et al.[31,55-57] |
| Moist Swabbing | Swab moistened with distilled water or saline | Smooth plastic, metal, glass | High | Enhances DNA uptake from slick surfaces | Hedman, et al. Gray & Passmore [7,58] |
| Double Swabbing | First swab moistened, second dry to collect residue | Non-porous like glass, steel, leather | High | Effective when contact time is short | Pang & Cheung, Hedman, et al. |
| Tape Lifting | Adhesive tape pressed and lifted to collect DNA | Glass, metal, plastic (flat surfaces) | Moderate | Non-invasive, quick, but may impair extraction | Comte, et al., Bonsu et al. |
| Scraping | Using scalpel/razor to scrape DNA material | Rough non-porous (brushed metal) | Moderate-High | Useful when swabbing fails | Coble & Butler, Sood & Gautam |
| FTA Card Transfer | Surface wiped or pressed onto FTA card | Delicate metal, plastic | High | Long-term preservation; chemical lysis included | Martin & Cotter, Alketbi [13] |
| Vacuum Collection | Suction of particles into filter (M-Vac or similar) | Larger or irregular non-porous (e.g. car body, weapons) | High | Best for wide areas, hard-to-reach spots | Li, et al., Nimbkar & Bhatt |
| Electrostatic Lifting | Charged film attracts DNA particles from surface | Smooth, fragile, or sensitive non-porous surfaces | High | Non-contact collection; suitable for fragile items [59] | Replogle & Andrews (2020), Tozzo, et al. (2022) [31,60] |
Cost-effectiveness analysis of collection methods
One important consideration largely overlooked in prior literature is the cost-effectiveness of different DNA collection methods. This analysis can help forensic labs make informed decisions based on their budget constraints and case requirements (Table 8).
| Table 8 | ||||
| Collection Method | Equipment Cost | Per-Sample Cost | Personnel Training Required | Cost-Effectiveness Ratio |
| Dry Swabbing | Low ($) | Low ($) | Minimal | High |
| Moist Swabbing | Low ($) | Low ($) | Minimal | High |
| Double Swabbing | Low ($) | Low ($) | Medium | Medium-High |
| Tape Lifting | Low ($) | Low ($) | Medium | Medium-High |
| Scraping | Low ($) | Low ($) | High | Medium |
| FTA Card Transfer | Medium ($$) | Medium ($$) | Medium | Medium |
| Vacuum Collection | High ($$$) | Medium ($$) | High | Low-Medium |
| Electrostatic Lifting | High ($$$) | Medium ($$) | High | Low-Medium |
Scalability and field application considerations
For forensic applications, the ability to scale methods for field application is critical [61] (Table 9):
| Table 9 | ||||
| Collection Method | Portability | Field Usability | Storage Requirements | Contamination Risk |
| Dry Swabbing | High | High | Minimal | High |
| Moist Swabbing | Medium | Medium | Requires buffer solution | Medium |
| Double Swabbing | Medium | Medium | Requires buffer solution | Medium-High |
| Tape Lifting | High | High | Minimal | Low |
| Scraping | Medium | Medium | Sample containers | High |
| FTA Card Transfer | High | High | Minimal (cards are stable) | Low |
| Vacuum Collection | Low | Low | Specialized filters | Medium |
| Electrostatic Lifting | Low | Low | Specialized containers | Low |
DNA extraction methods (Table 10)
| Table 10: Comparative analysis of extraction methods. | ||||
| Extraction Method | Description | Suitability for Non-Porous Surfaces | Equipment Cost | Time Requirement |
| Chelex Method | Uses Chelex resin with heat to lyse cells | Suitable for trace DNA on glass/plastic, not best for large DNA quantities | Low ($) | 1-2 hours |
| Silica-Based Bead Method [62] | Silica beads bind DNA in chaotropic salt | Highly effective for metal and glass, high DNA yield | Medium ($$) | 2-3 hours |
| DNA IQ | Magnetic bead-based DNA capture [63,64] | Ideal for metal/plastic, high efficiency for small samples | High ($$$) | 1-2 hours |
| Soaking Method | Soak surface then filter DNA from solution | Suitable for low-yield surfaces, but less efficient for high-yield samples | Low ($) | 4–24 hours |
Extraction efficiency vs. DNA integrity
Selecting extraction methods requires balancing between efficiency (maximum DNA recovery) and maintaining DNA integrity for downstream analysis [65] (Table 11):
| Table 11 | |||||
| Extraction Method | DNA Recovery Efficiency | DNA Integrity Preservation | Best for Low Copy Number | PCR Inhibitor Removal [66] | Automation Potential [67] |
| Chelex Method | Medium | Medium-Low | Yes | Medium | Low |
| Silica-Based Bead Method | High | High | Yes | High | High |
| DNA IQ | High | High | Yes | High | High |
| Soaking Method | Low-Medium | Low | No | Low | Low |
In conclusion, the extraction of DNA from non-porous surfaces presents unique challenges, because these surfaces, while not absorbing biological material like porous surfaces, often retain DNA in a dispersed and fragile state. Among the various methods evaluated, the Chelex method is suitable for trace amounts of DNA but often yields lower DNA quantities, making it effective for small, uncontaminated samples. The silica-based bead method offers superior DNA recovery, especially for non-porous surfaces such as glass and metal, where it provides higher DNA yields and is particularly useful for low-concentration samples. The DNA IQ method, utilizing magnetic beads, is also highly effective for non-porous surfaces, efficiently capturing DNA from challenging surfaces. Lastly, while the soaking method is less effective in terms of efficiency, provides a viable option for collecting DNA from surfaces with lower yields, although it may not perform well with larger or more concentrated deposits. Based on our comparative analysis, we suggest:
- For routine laboratory work, Moist swabbing combined with silica-based extraction offers the best Balance of cost, efficiency, and DNA quality.
- For critical evidence with minimal DNA, Double swabbing with DNA IQ extraction maximizes Recovery
- For field collection in remote locations, FTA cards provide stable storage and simplified processing.
- For large surface areas: Vacuum collection combined with silica-based extraction maximizes surface coverage and DNA yield.
Our cost-effectiveness analysis reveals that while advanced methods like vacuum collection and Electrostatic lifting offers superior recovery in specialized situations, but their high equipment costs and training requirements make them impractical for routine use in many forensic laboratories. The development of more affordable versions of these technologies represents an important direction for future research [68,69].
Future scope
In the last decade, the recovery of touch DNA from non-porous surfaces underwent a noteworthy development. Nonetheless, several options still remain for future advancement.
Steps towards the uniformity of protocols within different surfaces classification
Protocols for DNA retrieval from non-porous glass, plastic or metal materials need to be developed and accepted across different fields. For example, differences in texture, weathering, and sample age should be controlled to evaluate their effect on DNA recovery efficiency scalable to all levels [70,71].
Refined hybrid collection methods
Future research can focus on enhancing overall DNA collection from large or complex surfaces through combining moist swabbing followed by tape lifting or vacuum collection. Single workflows have the potential to be more effective than multiple ones and may be employed in the form of hybrid protocols [72,73].
Smart automated devices for DNA recovery
Robots or sensors can be utilized in the construction of DNA recovery tools. This method could reduce contamination compared to traditional approaches while improving precision of retrieval from fragile surfaces such as electronic devices, or valuable historical artifacts [74,75].
Diverse non-porous surface specific DNA adhesion studies
Protocols might be produced custom buffers designed for specific surfaces to improve DNA recovery efficiency, but only if further examined how non-porous materials at the molecular level interact with DNA [76].
Touch DNA recovery from digital devices
Research should concentrate on improving retrieval strategies for mobile gadgets like smartphones, as well as touchscreens, laptops, and other gadgets, Since DNA on these devices is often degraded or challenging to recover [77,78].
Renewable resources and studies conducted on the longevity of DNA
Research for forensic purposes on the combination of ultraviolet rays, humidity, temperature, and dust, with time on the non-porous surfaces of DNA, that is, Formex, could aid in estimating the realistic possibilities and viability of the DNA [79,80].
Detection and quantification of DNA in real time
Investigate the incorporation of real-time sensors or portable quantification gadgets to enhance the analysis of crime scenes and minimize the loss of samples before, during emergency transport, or storage facilities [20,81-94].
Amplified use of FTA Cards and the use of electrostatic devices
Electrostatic film lifting techniques require further optimization and FTA cards for after-the-fact forensic evidence, even with cold cases and delicate materials that must be preserved for DNA analysis [95].
Forensics training for awareness of new technology
With evolving techniques, field staff training must be improved to ensure accurate, modern collection, minimal cross-contamination, and advanced tools in new surfaces usually encountered in crime scenes [96].
The authors declare that no external funding was received for this work. The research was independently conducted. The authors extend their gratitude to colleagues and peer reviewers for their valuable feedback during manuscript development.
- van Oorschot RA, Ballantyne KN, Mitchell RJ. Forensic trace DNA: a review. Investig Genet. 2010;1(1):14. Available from: https://doi.org/10.1186/2041-2223-1-14
- Gill P. Touch DNA: a challenge to forensic science. Forensic Sci Int Genet. 2011;5(1):1–3.
- Sutherland CS, Bell S. Optimizing DNA collection from non-porous surfaces. J Forensic Sci. 2012;57(6):1420–1424.
- Smith M, Johnson K. Forensic vacuum collection of touch DNA. Forensic Sci Rev. 2018;30:105–110.
- Singh V, Kumar M. The role of genetic profiling in forensic investigations. J Forensic Genet. 2020;5:24–29.
- Gill P, Solsberg L. Low copy number DNA analysis and the interpretation of complex cases. Forensic Sci Int Genet. 2010;4(2):92–96.
- Gray S, Passmore D. Environmental degradation of DNA on surfaces and forensic implications. Forensic Sci Int. 2018;292:37–47.
- Krane DE, Lund SP. Commentary: Misuse of DNA evidence in criminal cases. Forensic Sci Int Genet. 2016;24:194–195.
- Kumar P, Bhandari D, Chouhan JS, Sahajpal V. Touch DNA analysis in forensic science: a review. Egypt J Forensic Sci. 2023;13(1).
- Li R. Forensic biology. 2nd ed. Hoboken (NJ): Wiley; 2015.
- Qureshi H, Lal M. Touch DNA persistence on frequently handled objects. J Forensic Stud. 2019;22(3):89–95.
- Chatterjee A, Josan A. Best practices for handling touch DNA at outdoor scenes. Field Forensics Man. 2021;11(2):105–110.
- Alketbi SK, Goodwin W. Touch DNA collection techniques for non-porous surfaces using cotton and nylon swabs. JSTR. 2021;36. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4368493
- Brown R. Swab-based methods for DNA recovery from smooth surfaces. Sci Justice. 2012;50:256–263.
- Zhang T, Dong X, Lin W. Quantitative approaches to evaluate DNA recovery from non-porous materials. Forensic Biol Rev. 2022;11(3):150–156.
- Sekhon G, Dey N. Collection tool variability in trace DNA results. Int J DNA Tools Tech. 2021;7:50–56.
- Sinha S, Yadav B, Bumbrah GS. Potential utility of Touch DNA in forensic investigations. J Indian Acad Forensic Med. 2021;43(2).
- McGill E, Singh J. Dual-swab approaches for non-porous evidence collection. J Evid Tech. 2022;7(1):91–96.
- O'Connor B, Malik T. Trace DNA collection in wet environments. Forensic Field Cond Stud. 2019;6:35–40.
- Franco JM, Dias S. Comparative analysis of sample carriers in trace DNA preservation. Forensic Collect Tools Rev. 2020;2:12–17.
- Dev R, Mishkin T. Touch DNA persistence post-cleaning of surfaces. J Forensic Surf Sci. 2019;9:58–63.
- Khan MI, Sharma V. Innovations in swab design for trace DNA recovery. Forensic Evid Technol. 2020;4(2):33–38.
- Li R. Principles of forensic DNA typing. 3rd ed. Boca Raton (FL): CRC Press; 2018.
- Simpson G, O'Hara B. Effectiveness of electrostatic lifting for touch DNA recovery. Forensic Sci Int Genet. 2014;7:34–40.
- Green DA, Patel LM. Environmental impacts on touch DNA stability. Forensic DNA Rep. 2017;8:43–49.
- Butler JM. Fundamentals of forensic DNA typing. Gaithersburg (MD): NIST; 2010.
- Burrill J, Daniel B, Frascione N. A review of trace ‘touch DNA’ deposits: Variability factors and an exploration of cellular composition. Forensic Sci Int Genet. 2018;37:165–173. Available from: https://www.fsigenetics.com/article/S1872-4973(18)30274-6/abstract
- Sharma K, Yadav R. Swabbing and tape lifting for touch DNA recovery: A comparative study. Forensic Investig J. 2021;28:114–121.
- Zhou H, Tran D. Polymer-based substrates for improved DNA retention. Forensic Polym Sci Lett. 2020;7:61–66.
- Ali M, Verghese R. Evaluation of surface material chemistry on touch DNA adherence. J Surf Forensic Chem. 2022;9:44–50.
- Tozzo P, Mazzobel E, Mazzobel B, Mazzobel A, Mazzobel L. Touch DNA sampling methods: Efficacy evaluation and systematic review. Int J Mol Sci. 2022;23(24). Available from: https://doi.org/10.3390/ijms232415541
- Wallace TM, Franks J. Surface contamination and touch DNA: A forensic risk analysis. Forensic Res Criminol Int J. 2019;6(2):23–28.
- Coble MD, Butler JM. DNA typing methods for crime scene investigation. Forensic Sci Int. 2002;101(2).
- Prasad L, Rani S. Forensic implications of long-term surface contact DNA. Trace DNA J. 2020;5(2):58–64.
- Bogen S, Marchionda G. Techniques for recovering DNA from smooth surfaces. Forensic Sci Int. 2015;199(1).
- Rahman A. Best practices for storing and handling trace DNA evidence. J Forensic Pract. 2017;11:133–139.
- Tan C, Ahmed R. Degradation of touch DNA under varying humidity conditions. Forensic Sci Int Genet Suppl Ser. 2020;8:90–94.
- Fraser H, Dillon L. Swab elution volume and its impact on DNA concentration. Genet Sampl Sci. 2018;6(3):79–84.
- Ibrahim F, Thomas E. Air-drying vs heat-drying effects on trace DNA yield. Forensic Drying Tech Rev. 2019;2(2):15–20.
- Govind N, Sengupta A. Nano-structured swabs for improved DNA pickup. J Forensic Nanotech. 2021;1(1):10–14.
- Mishra RS, Srivastava AS. Challenges in DNA recovery from non-porous surfaces. J Forensic Sci. 2016;51(4).
- Cooper JA, Lin RS. A comparative review of vacuum vs swab-based DNA recovery. Forensic Sci Law J. 2021;7:19–26.
- MacIntyre C, Singh A. Electrostatic lifting film vs adhesive tape in trace DNA recovery. J Forensic Mater. 2021;12(1):11–17.
- Han Y, Lee S. Microfluidic DNA extraction from hard surfaces. Forensic Sci Rep. 2022;14:67–72.
- Zhang Y, Malik S. Comparison of lyophilized vs liquid buffer for field DNA recovery. Adv Forensic DNA Tools. 2021;3:90–95.
- Oliveira JP, Mendes FR. Swabbing techniques for textured non-porous materials. Forensic Tools Tech. 2016;6:100–105.
- Deshmukh A, Rao T. Pre-moistened vs dry swab effectiveness on glass. J Investig Forensics. 2018;19:58–63.
- Bakshi A, Kapoor D. Cell collection efficacy in trace DNA recovery: Dry vs moistened swabs. J Biol Evid Tech. 2022;4(2):50–55.
- Singh N, Kapoor M. Stability of DNA samples stored at ambient temperature. J DNA Preserv. 2021;4:75–80.
- Wang L, Zhao Y. DNA degradation on smooth surfaces: A forensic perspective. J Forensic Legal Med. 2018;24:78–83.
- Singh H, Kaur N. Direct collection and analysis of touch DNA from glass surfaces. Forensic Sci Rev. 2019;25:120–126.
- Kaur R, Mehta D. Challenges of recovering touch DNA from textured non-porous surfaces. Int J Forensic Sci. 2018;14(1):55–60.
- West M, Gibson R. Tape lifting versus swabbing for touch DNA collection from non-porous surfaces. Sci Justice. 2016;52:87–91.
- Iyengar R, Kapoor P. Moisture control in storing touch DNA evidence. Forensic Storage Handling. 2021;5:10–16.
- Ridgway L. DNA recovery from plastic: A critical review. J Forensic Legal Med. 2015;18:64–70.
- Leone C, Byrne M. Method validation for non-porous surface DNA collection. Forensic Methods Validat. 2019;4:90–96.
- Subramanian K, Pillai R. Quantitative study on DNA recovery efficiency from ceramic surfaces. J Adv Forensic Res. 2020;8:33–38.
- Xu T, Lopez R. Role of surface texture in DNA yield from glass and plastic. Int Forensic Res Lett. 2019;10:15–20.
- Pandey S, Raj A. Impact of sampling pressure on DNA yield from smooth surfaces. Forensic Stud Int. 2020;9:62–68.
- Nascimento JL, Silva C. UV exposure and DNA degradation on smooth surfaces. Forensic Light Source J. 2020;6(1):33–37.
- Das M, Verma N. Comparison of DNA collection buffers for field use. Pract Forensic Appl. 2020;9:44–49.
- VanderWaal K. DNA extraction from difficult substrates: The silica method. J Forensic Sci. 2015;60(1).
- Steele CH, Brown DW. Use of magnetic beads for DNA extraction from smooth surfaces. Forensic Sci Rev. 2015;29:205–210.
- Hargrove PC, Fischer G. Magnetic bead-based extraction methods for touch DNA. Forensic Sci Int. 2020;311:73–78.
- Lee JP, Chouhan S. Review of DNA extraction methods from non-porous surfaces. Forensic Sci J. 2016;33(1):40–47.
- Prasad N, Mishra L. Use of direct PCR in touch DNA profiling. J Forensic Technol. 2017;15:72–76.
- Mathews BL, Ramesh D. Automated systems for touch DNA profiling. Forensic Sci Autom. 2019;8:57–62.
- Palkovits PD. Electrostatic lifting: A new frontier for touch DNA. Forensic Sci Int Genet. 2014;4:254–257.
- Patel R. Silica-based DNA extraction: The gold standard. Forensic Sci J. 2016;10:144–151.
- Bennet A, Lee P. Swab texture and material composition in forensic sampling. Mater Sci Forensics. 2020;6:40–46.
- Naidu M, Rajput P. Field sampling protocols for high-throughput trace DNA. Forensic Process Eng J. 2021;3(2):81–86.
- Thompson R, Ali H. Decontamination protocols prior to DNA swabbing. Forensic Cleanliness Rep. 2018;5:19–24.
- Tanaka M, Ko Y. Innovative adhesives for tape lifting of trace DNA. Forensic Adhes Tech Rev. 2021;5:62–67.
- Zhang T, Dong X. Recovery of touch DNA from electronics and mobile devices. J Forensic Sci Technol. 2019;32.
- Anderson M. Challenges in extracting touch DNA from metal objects. Forensic Sci Int. 2017;220:45–49.
- Menon V, Thomas D. Efficiency of various buffer systems in touch DNA extraction. Biotech Forensics. 2018;13:39–45.
- Das R, Pillai S. Vacuum-based DNA collection in field investigations. Forensic Sci Int Tech Tools. 2021;7:93–98.
- Harshavardhan R, Patel M. Evaluating DNA persistence on mobile devices. Mobile Forensic Stud. 2021;4(1):23–28.
- Parker D, Zhang Y. Humidity and temperature effects on DNA swab yields. Environ Forensic Rev. 2021;13(1):25–30.
- Varma A, Joshi K. The forensic value of partial DNA profiles. J Forensic Pract. 2022;12(2):112–118.
- Akhtar Z, Fernandes M. Quantification of touch DNA using qPCR. J Genet Forensics. 2018;17:88–94.
- Watson P, Kim J. Stabilizing agents in DNA transport from field to lab. Forensic Handl Tech. 2020;10(2):49–54.
- DeSilva T, Nair A. Direct PCR success rate on various non-porous substrates. J Forensic Genet. 2021;21:101–107.
- Choi M, Yu L. Limitations in swab recovery efficiency across surface types. J Forensic Sampling. 2017;6(3):71–76.
- Patel SK, Trivedi N. Biofilm formation impact on forensic DNA evidence. Forensic Microbiol Rep. 2020;5:33–38.
- Gonzales ER, Mendes L. Factors affecting PCR inhibition from smooth surface residues. Genet Evid Res. 2018;11:80–85.
- Cheung S, Park H. Optimizing storage conditions for DNA swabs. Evid Preserv J. 2020;7:43–48.
- Prakash R, Saxena A. Portable DNA kits for field touch DNA recovery. J Rapid Forensic Methods. 2022;3:70–75.
- Rahman T, Saha V. Storage tube contamination risks in touch DNA workflows. J Forensic Lab Pract. 2020;6(1):20–25.
- Srivastava A, Desai R. Microbial contamination impact on trace DNA analysis. Biocontam Forensics. 2021;5(3):61–66.
- Pathak D, Anand R. Low-copy DNA recovery methods: Field versus lab comparisons. Compar Forensic Sci. 2021;6(2):88–93.
- Kumar R, Lalwani A. Statistical modeling of touch DNA yield variation. Forensic Prob Anal. 2021;4(1):22–27.
- Mehra S, Sen K. Cleaning agents and their interference with trace DNA detection. Forensic Chem J. 2022;5(1):30–34.
- Stover S. Comparative analysis of DNA extraction methods for non-porous surfaces. J Forensic Res. 2021;9(4).
- Boyd P, Smith G. Use of FTA cards in touch DNA recovery. J Forensic DNA. 2017;9(2):102–107.
- Goyal P, Nanda R. Cross-contamination risks in multi-touch forensic surfaces. J Crime Scene Contam Stud. 2021;3:88–92.
- Noyes JM. DNA recovery from difficult substrates in forensic investigations. Forensic Sci Rev. 2017;23(2).
- Binns LA, McCormick PH. DNA extraction from non-porous materials: Best practices. Forensic DNA Rev. 2015;16.
- Amsel T. Optimizing collection and extraction methods for touch DNA on glass. FSI: Genetics. 2020;5:12–18.
- Dube J, Kumar S. Enhancing DNA recovery from metal surfaces. Int J Forensic Sci. 2019;19:88–92.