More Information
Submitted: March 10, 2023 | Approved: March 27, 2023 | Published: March 28, 2023
How to cite this article: Singh B, Nailkar SR, Badkhambhekar CA, Prajapati S, Kaur S. Development of qualitative GC-MS method for simultaneous identification of PM-CCM a modified illicit drugs preparation and its modern-day application in drug-facilitated crimes. J Forensic Sci Res. 2023; 7: 004-010.
DOI: 10.29328/journal.jfsr.1001043
Copyright License: © 2023 Singh B, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: GC-MS; PM-CCM; Pregabalin; Methamphetamine; Mirtazepine; Clonazepam; Caffeine
Development of qualitative GC MS method for simultaneous identification of PM-CCM a modified illicit drugs preparation and its modern-day application in drug-facilitated crimes
Bhagat Singh*, Satish R Nailkar, Chetansen A Bhadkambekar, Suneel Prajapati and Sukhminder Kaur
Central Forensic Science Laboratory (CFSL), Ministry of Home Affairs, Goverment of India, Gat No. 6, Nanoli Tarafe Chakan, MIDC Road Talegaon, Pune, Maharashtra, 410507, India
*Address for Correspondence: Bhagat Singh, M.Sc, Department of Chemical Sciences, Central Forensic Science Laboratory (CFSL), Ministry of Home Affairs, Goverment of India, Gat No. 6, Nanoli Tarafe Chakan, MIDC Road Talegaon, Pune, Maharashtra, 410507,India, Email: [email protected]
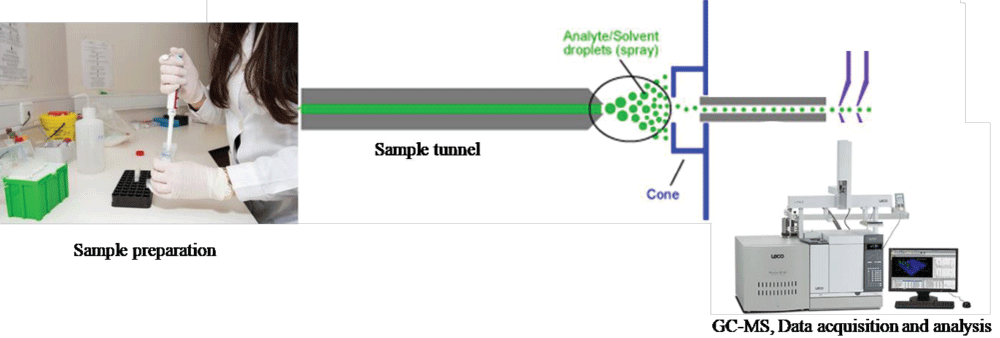
Prescriptions for psychoactive substances such as Pregabalin, Methamphetamine, Caffeine, Clonazepam and Mirtazapine (PM-CCM) are common in the treatment of a variety of disorders. Indeed, the PM-CCM has been used in different therapeutic areas, including insomnia, anxiety, seizure disorders, etc. Unfortunately, these psychoactive substances are present in the illegal street market, leading to a lot of drug abuse among some addicted users, road insecurity and suicide. Hence, it has become essential to validate and develop a rapid and effective method to analyze the PM-CCM, a modified illicit drug, for drug abuse in the forensic sciences. A simple, rapid, specific and sensitive Gas Chromatography-Mass Spectrometry(GC-MS) method has been developed for the identification of Pregabalin, Methamphetamine, Caffeine, Clonazepam and Mirtazapine (PM-CCM) in forensic exhibits. At room temperature, the sample was ultrasonicated for 5 minutes before being extracted with methanol. A highly precise auto-injector is used to inject a very small quantity of samples for analysis. Helium is used as a carrier gas with a flow rate of 1 ml/min. The separation of PM-CCM was performed on SH-RXi-5 MS, ID.25 mm, film thickness. 25 µm, length of 30 m column. The constituents of PM-CCM were identified by the mass-to-charge ratio (m/z ratio) of fragments of the parent compound by comparing them with the NIST-17 MS Library. Separation and identification of PM-CCM were achieved within a 15-minute run. The proposed method has been successfully used for the routine analysis of PM-CCM in complex illicit drug preparations and in forensic exhibits as well. The application of above discussed qualitative analysis method and screening of PM-CCM, modified illicit drug samples demonstrates the potential and applicability of the technique to the fast chemical profiling of illicit samples.
Graphical abstract:
The impact of illicit drug markets on the incidence of violence varies tremendously depending on many factors. Drug abuse is a very serious threat to human society and it’s increasing day by day, posing a major challenge to drug analysts. According to the United Nations Office on Drugs and Crime (UNODC) [1], drug use is increasing at an alarming rate, with approximately 275 million people using drugs in 2019, up to 22 percent from 2010 and approximately 500,000 people dying as a result of drug users that year Azimi and Docoslis [2]. Consumption of illicit drugs is increasing day by day globally and emerging as a public health issue and has pernicious impacts on the consumer’s health Crowley, et al. [3], Joye, et al. [4], Vearrier [5]. Clandestine producers used a variety of ways to modify the drugs of abuse by mixing adulterants for economic gain and escaping the eyes of investigating agencies. Adulterants play a significant role in the masking of drugs, making them difficult to identify in forensic exhibits. Adulteration depends on chemical additives and cutting agents available in local markets. Some local anesthetic compounds, medicinal plant extracts and non-narcotic medicines have been used to boost narcotic potential and produce synergistic effects with illicit drugs. Pregabalin and caffeine are the adulterants of choice in drug abuse due to their euphoric effect. The euphoric effect of Pregabalin was reported by Zaccara, et al. 2012 and Pfizer, 2016 Zaccara, et al. [6], and Pfizer [7]. Pregabalin is a structural analogue of aminobutyric acid, the primary inhibitory neurotransmitter in the mammalian central nervous system; both are used to treat epileptic seizures and neuropathic pain Hlozek, et al. [8]. It is chemically known as (S)-2(amino methyl)-5-methyl hexanoic acid and is a white crystalline solid, which is soluble in water and in both basic and acidic aqueous solutions Donald [9], Warner and Figgitt [10]. Caffeine, levamisole and procaine, local anesthesia, was found with several drugs of abuse such as heroin, fentanyl and cocaine Fiorentin, et al. [11]. Separation, isolation and identification of constituents of drug mixtures are tedious tasks. However, common methodologies for analysis and standard reporting practices frequently do not include cutting agents, resulting in a lack of or inadequate information regarding the prevalence of these substances. Several color tests are available for the indicative presence of functional groups like alkaloids, cannabinoids, carbonyls and some groups of unsaturated compounds (Clarke’s Analysis of Drugs and Poisons [12], Third edition). Numerous techniques have been developed so far for illicit drug detection in forensic toxicology, such as nuclear magnetic resonance Assemat, et al. [13], Burns, et al. [14]. A previous research study has revealed that only a few instrumental methods are available for the identification of some chemical constituents of locally modified illegal drug preparations. High-Performance Liquid Chromatography with UV detectors is now the most commonly used method for estimating Pregabalin in bulk capsule dosage form and pharmaceutical formulations Seema, et al. [15], Gujral, et al. [16]. Similarly, spectrophotometric methods are also used for the determination of pregabalin content in bulk and dosage forms Patil, et al. [17]. Liquid Chromatography-Mass Spectrophotometry (LC-MS), LC with fluorescence detection was also used to determine Pregabalin in human plasma and serum Mandal [18], Oertel [19], Vermeij [20]. Pavlova et al, have estimated that amphetamine, methamphetamine, and caffeine are in tablet form simultaneously, Pavlova, et al. [21]. Jun, et al. [22] performed chiral capillary zone electrophoresis coupled with acetonitrile field amplified separation of Mirtazapine, N-Demethylmirtazepine, 8-Hydroxymirtazepine and Mirtazapine-N-oxide. Labat, et al. [23] developed spectrofluorimetric, spectrofluorimetric, HPLC and CZE. However, all these available methods are expensive and time-consuming pretreatment processes. Apart from this, these methods pose serious health-threatening exposure to chemical solvents during derivatization and laborious clean-up procedures for the analysis of samples. As a result, there is an immediate need for a robust and distinct analysis method for detecting novel PM-CCM in adulterated illicit drug preparations. The development of rapid, accurate, and affordable drug detection and identification methods could be a powerful tool in our efforts to reduce the overwhelming effects stemming from illicit drug use, or more generally, drug abuse. Therefore, in this study, the author is keen to develop a GC-MS-based qualitative method for the identification of this illicit drug preparation of forensic interest. Hence, an attempt has been made to develop a simple, rapid, specific, and sensitive method for the concurrent analysis of constituents of PM-CCM.
Reagents and chemicals
The chemical standards and solutions used in this study for sample extraction and dilution procedures were purchased from SRL, Pvt. Ltd., India, and Fisher Scientific, Pvt. Ltd., India. Standard reference materials for drugs are procured from the Indian Pharmacopeia, Ministry of Health & Family Welfare, Govt. of India, Ghaziabad.
Preparation of samples
Regarding GC-MS experiments, all samples were prepared with 10 mg each of methamphetamine, Mirtazepine, Clonazepam, Pregabalin and Caffeine powder homogenized well, extracted with 3 x 5 ml methanol and ultra-sonicated for 5 minutes at room temperature. The homogenized extract was filtered through Whatman filter paper No. 1 and a final volume of 10 ml was made up by concentrating at room temperature. An experiment was conducted in order to evaluate the minimal number of acquired spectra in order to minimize the variability and therefore to perform accurate identification.
Preparation of standards
The stock solutions of each of Pregabalin, Methampheta-mine, Mirtazepine, Clonazepam and caffeine were prepared at 1 mg/ml concentration in methanol and sonicated for 5 minutes at room temperature. The homogenized extract was filtered through man’s filter paper No 1 and the working standard solutions were prepared by diluting the standard solutions to the appropriate concentrations as needed. The working standard solutions were stored at −20 °C when not in use, and they were prepared monthly.
Instrumentation and experimental conditions
A GC-MS model QP2020NX Shimadzu equipped with a high-quality AOC-20i plus auto-injector was used. The column was 30 m SH-RXi-5 MS with a 0.25 mm ID and 0.25 µm film thickness. At a constant flow of 1 ml/min, helium was used as a carrier gas. A split less injection was used. The injector and interface line temperatures were kept constant at 260 and 220 degrees Celsius, respectively. The 0.5 µl sample was injected by the high-quality AOC-20i plus auto-injector to the preconditioned GC-MS-QP2020NX Shimadzu for recording the total ion chromatogram and mass spectra of compounds under the following operating parameters: oven temperature of 90 °C held for 1 min, ramp @25 °C/min up to 280 °C hold for 7 min. The MS conditions were kept at 70 eV ionization energy, 200 °C ion source temperature, and a mass range of 40 - 550 eV.
Compounds identification
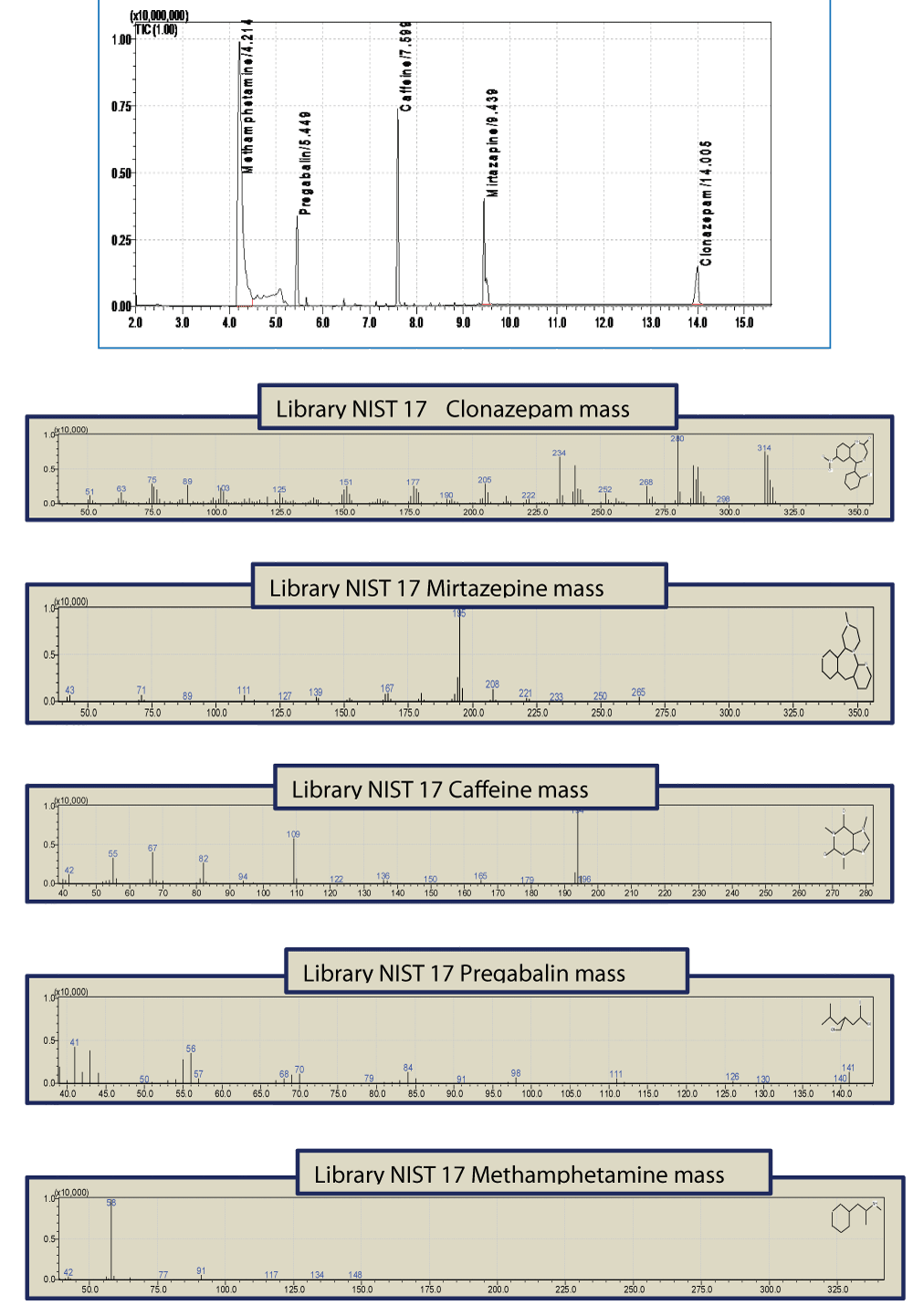
For data acquisition and processing, the GCMS solution, version 4.50 software, was used. The results were screened using the library of the National Institute of Standards and Technology (NIST-17 MS Library) and compared with the mass spectra of standard reference materials Figure 1.
Figure 1: A typical TIC chromatograph of a sample containing a mixture of Methamphetamine, Pregabalin, Caffeine, Mirtazepine and Clonazepam of a concentration of 1 mg/ml.
Method validation
Method validation was performed in order to evaluate the method in terms of LOD, precision, accuracy, specificity, robustness and system suitability.
The analytical procedure was evaluated and proved to be applicable for illicit drug identification. This method helped in avoiding contamination from derivatizing reagents because derivatization was excluded. The obtained coefficients indicated the linearity of the results and an excellent correlation between concentration and response for each drug standard was produced. Sensitivity and accuracy are dynamic factors in drug profiling procedures because any disparity in chromatograms can affect the interpretation of the comparison process. Therefore, the accuracy of the entire method was investigated by the precision of RT, where sensitivity was measured by the Limit of Detection (LOD). The LOD was determined to be the lowest concentration, yielding an integrated height corresponding to three times the height measured after injection of each drug standard.
Specificity and sensitivity
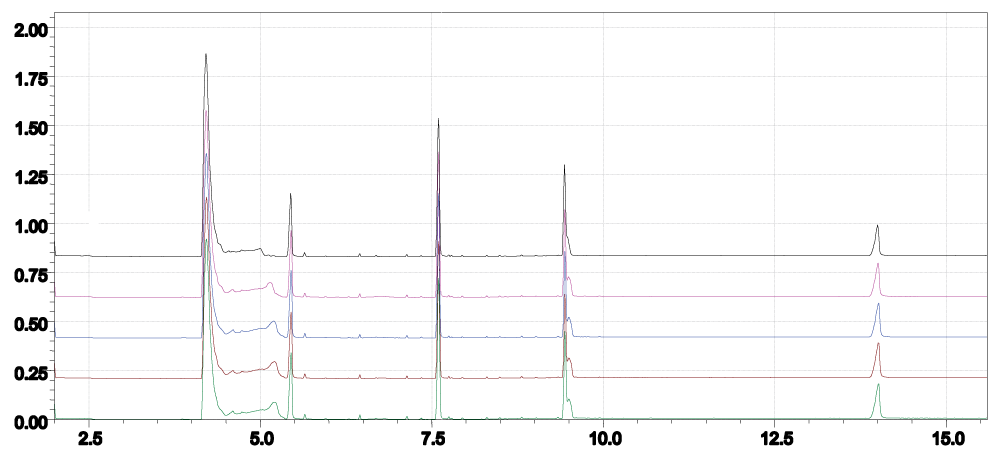
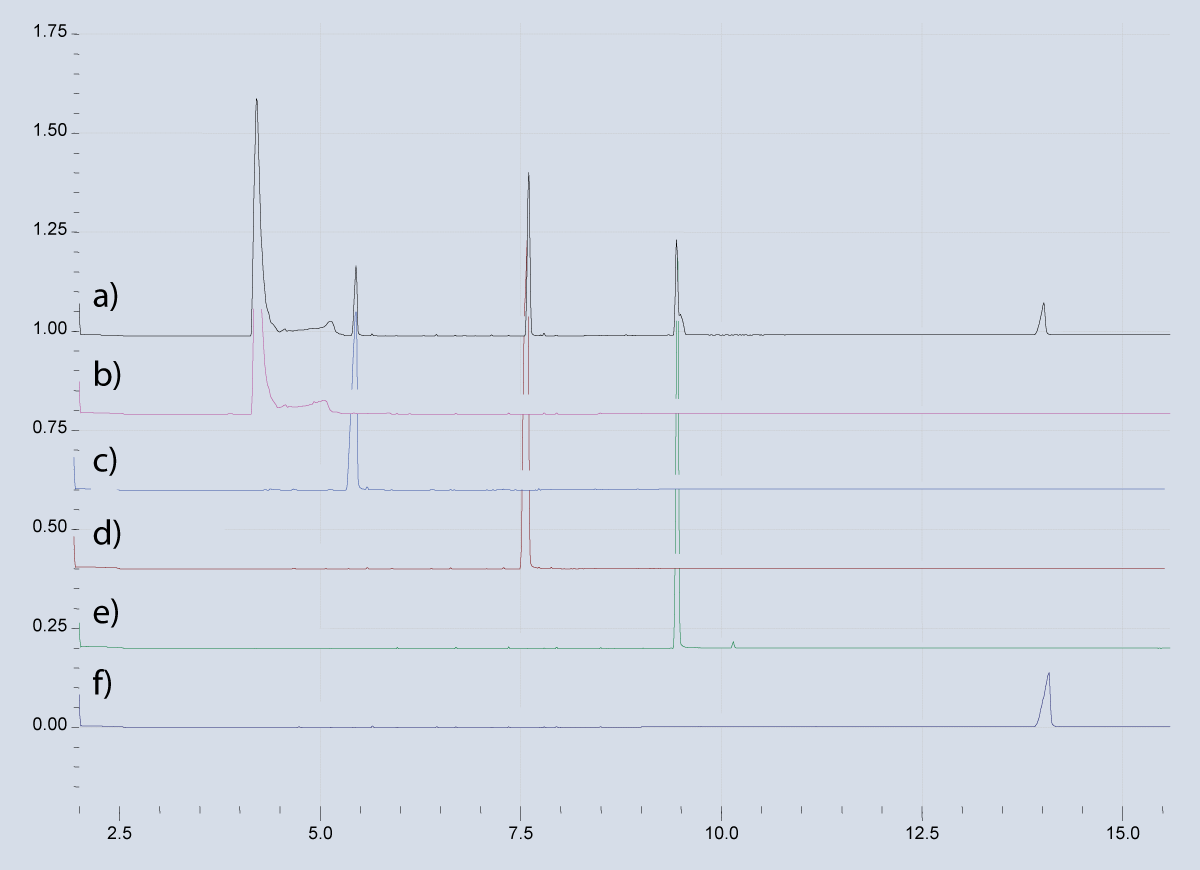
The specificity of the GC-MS method has been demonstrated by the representative chromatograms for Methamphetamine, Pregabalin, Caffeine, Mirtazepine and Clonazepam in the sample (Figure 2). The sample showed no significant interference with other compounds at retention times compared with standard compounds (Figure 3). The Limit of Detection (LOD) of an individual analytical procedure is the lowest amount of analyte in a sample that can be detected but not necessarily quantified as an exact value. The minimum detectable concentration (LOD) of this method, based on the observation method for TIC mode, was in the range of 1.95 ng/mLto 10 ng/mLfor Methamphetamine (1.95 ng/ml), Pregabalin (2.5 ng/ml), Caffeine (0.60 ng/ml), Mirtazepine (8.0 ng/ml), and Clonazepam (10 ng/ml).
Figure 2: Chromatograph of replicate samples containing a mixture of Methamphetamine, Pregabalin, Caffeine, Mirtazepine and Clonazepam.
Figure 3: Chromatograph of a) sample b) Std. Methamphetamine) Std. Pregabalin d) std. Caffeine e) Std. Mirtazepine f) Std. Clonazepam.
Precision and accuracy
The precision of the analytical method was determined by repeatability (intra-day) and intermediate precision (inter-day) using five replicates for both samples and standards. The results show that the method was reproducible and reliable within the acceptable analytical range (Table 1).
| Table 1: The sample's RT in relation to the compound standards. | ||
| RT detected for the sample, min a | ||
| Compound | Interday | Intraday |
| Methamphetamine | 4.2 ± 0.0015 | 4.2 ± 0.0038 |
| Pregabalin | 5.4 ± 0.002 | 5.4 ± 0.0020 |
| Caffeine | 7.6 ± 0.0015 | 7.6 ± 0.0018 |
| Mirtazepine | 9.4 ± 0.0040 | 9.4 ± 0.0025 |
| Clonazepam | 14.0 ± 0.0120 | 14.0 ± 0.0034 |
| aEach value is the mean ± SD; n = 5. | ||
Robustness
Prepare the three samples and analyze them under the conditions by changing the flow rate by ± 0.1 ml, injector temperature ± 10 °C, detector temperature ± 10 °C and ms interface ± 10 °C. Evaluate the system suitability criteria in terms of retention time and mass spectra, no significant differences were observed in the results.
The PM-CCM sample was extracted with different solvents such as chloroform, ether, chloroform: ether (1:1), and methanol. Methanol was found to be the most suitable solvent for the extraction of PM-CCM. A highly precise auto-injector was used to inject a 0.5 µL sample into GC and a chromatogram was recorded. Chromatographic separation and RT value were recorded for each analyte and standards in scan mode and optimal separation of selected compounds was achieved within 15 min. The carrier gas flow and oven temperature were optimized in such a way that no two compounds overlapped or interfered with each other. The mean retention time for Methamphetamine, Pregabalin, Caffeine, Mirtazepine and clonazepam was optimized at 4.21 min, 5.45 min, 7.60 min, 9.44 min and 14.00 min, respectively (Table 1). The response for the 5 replicated samples resulted in relative standard deviations of 0.07% for Methamphetamine, 0.03% for Pregabalin, 0.01% for Caffeine, 0.05% for Mirtazepine and 0.12% for Clonazepam retention time. The GCMS solution, version 4.50 software, was used for data acquisition and processing. The results were screened using the library of the National Institute of Standards and Technology (NIST-17 MS Library) and compared with the mass spectra of standard reference materials. The described method is highly efficient with a simple extraction procedure and does not require sample derivatization.
A rapid and simple GC-MS method was developed and validated to identify complex illicit drug mixtures of forensic interest. This method showed good separation among the compounds’’ as in comparison with existing methods (e.g., spectrofluorimetric, HPLC and CZE), the newly proposed method provides its efficiency in separating various complex compounds without any significant interference with each other or other neutral substances’’. A qualitative analysis should precede pharmacological and toxicological studies and the GC-MS method developed here may be useful to rapidly identify designer drugs in legal highs. The results are reproducible and reliable for carrying out qualitative analysis on matrix samples. This analytical procedure may find wide application in the identification of other narcotic drugs as well as psychotropic substances in illicit drug preparations. The newly proposed method can provide useful information concerning the analysis of complex illicit drug mixtures of forensic interest.
The authors are thankful to the Director, CFSL, DFSS, Ministry of Home Affairs, Govt. of India, Pune and Chief Forensic Scientist, DFSS, Ministry of Home Affairs, Govt. of India, New Delhi for constant scientific support and encouragement in this study.
Article classification
Drug Trafficking, Forensic Chemistry, Crime Scene.
- Aberhausen UN. United Nations Office on Drugs and Crime. Resolutions and Decisions. 2018. www.unodc.org/unodc/en/commissions/CND/Resolutions_Decisions/Resolutions-Decisions.
- Azimi S, Docoslis A. Recent Advances in the Use of Surface-Enhanced Raman Scattering for Illicit Drug Detection. Sensors (Basel). 2022 May 20;22(10):3877. doi: 10.3390/s22103877. PMID: 35632286; PMCID: PMC9143835.
- Crowley R, Kirschner N, Dunn AS, Bornstein SS; Health and Public Policy Committee of the American College of Physicians; Abraham G, Bush JF, Gantzer HE, Henry T, Kane GC, Lenchus JD, Li JM, McCandless BM, Candler SG. Health and Public Policy to Facilitate Effective Prevention and Treatment of Substance Use Disorders Involving Illicit and Prescription Drugs: An American College of Physicians Position Paper. Ann Intern Med. 2017 May 16;166(10):733-736. doi: 10.7326/M16-2953. Epub 2017 Mar 28. PMID: 28346947.
- Joye T. Bararpour N, Augsburger M, Boutrel B, Thomas A. In situ metabolomic changes in rat hippocampus after acute cocaine administration. Int. J. Mass Spectrom. 2017; 437: 87–91; 10.1016/j.ijms. 2017;12:001.
- Vearrier L. The value of harm reduction for injection drug use: A clinical and public health ethics analysis. Dis Mon. 2019 May;65(5):119-141. doi: 10.1016/j.disamonth.2018.12.002. Epub 2018 Dec 29. PMID: 30600096.
- Zaccara G, Gangemi P, Perucca P, Specchio L. The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2011 Apr;52(4):826-36. doi: 10.1111/j.1528-1167.2010.02966.x. Epub 2011 Feb 14. PMID: 21320112.
- Pfizer Canada Inc. Lyrica® Product Monograph. December 6, 2016. http://www.pfizer.ca/sites/g/files/g10028126/f/201612/LYRICA_DC_PM_198215_6Dec2016_E.pdf
- Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, Nováková P, Šíchová K, Štefková K, Tylš F, Kuchař M, Páleníček T. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol. 2017 Dec;27(12):1223-1237. doi: 10.1016/j.euroneuro.2017.10.037. Epub 2017 Nov 10. PMID: 29129557.
- Donald JA. Medicinal Chemistry and Drug discovery (6th edn) Wiley, New Jersey. 2003; 312-313.
- Warner G, Figgitt DP. Pregabalin: as adjunctive treatment of partial seizures. CNS Drugs. 2005;19(3):265-72; discussion 273-4. doi: 10.2165/00023210-200519030-00007. PMID: 15740180.
- Fiorentin TR, Krotulski AJ, Martin DM, Browne T, Triplett J, Conti T, Logan BK. Detection of Cutting Agents in Drug-Positive Seized Exhibits within the United States. J Forensic Sci. 2019 May;64(3):888-896. doi: 10.1111/1556-4029.13968. Epub 2018 Nov 28. PMID: 30485426.
- Clarke’s analysis of drugs and poisons, 3rd edition. Eds. AC Moffat, MD Osselton and B Widdop. Pharmaceutical Press, London (UK). 2003; ISBN 085369 473 7.
- Assemat G, Balayssac S, Gerdova A, Gilard V, Caillet C, Williamson D, Malet-Martino M. Benchtop low-field 1H Nuclear Magnetic Resonance for detecting falsified medicines. Talanta. 2019 May 1;196:163-173. doi: 10.1016/j.talanta.2018.12.005. Epub 2018 Dec 7. PMID: 30683346.
- Burns NK, Theakstone AG, Zhu H, O'Dell LA, Pearson JR, Ashton TD, Pfeffer FM, Conlan XA. The identification of synthetic cannabinoids surface coated on herbal substrates using solid-state nuclear magnetic resonance spectroscopy. Anal Chim Acta. 2020 Apr 1;1104:105-109. doi: 10.1016/j.aca.2019.12.051. Epub 2020 Jan 21. PMID: 32106940.
- Seema A, Jeeja P, Ashish J. Development and Validation of HPLC Method for Estimation of Pregabalin in Bulk & Capsule Dosage Form. Pharm Anal Acta. 2016; 7: 492. doi:10.4172/2153-2435.1000492
- Gujral RS, Haque SM, Shanker P. Development and Validation of Pregabalin in Bulk, Pharmaceutical Formulations and in Human Urine Samples by UV Spectrophotometry. Int J Biomed Sci. 2009 Jun;5(2):175-80. PMID: 23675132; PMCID: PMC3614770.
- Patil DD, Patil MS, Wani YB. Spectrophotometric method for pregabalin determination: An experimental design approach for method development. Jour. of the Associ. of Arab Unive. for Bas. and Appl. Sci. 2018; 21:(21); 31-37.
- Mandal U, Sarkar AK, Gowda KV, Agarwal S, Bose A, Bhaumik U, Ghosh D, Pal TK. Determination of Pregabalin in Human Plasma Using LC-MS-MS. Chromatographia 2008; 67(3-4):237-243.
- Oertel R, Arenz N, Pietsch J, Kirch W. Simultaneous determination of three anticonvulsants using hydrophilic interaction LC-MS. J Sep Sci. 2009 Jan;32(2):238-43. doi: 10.1002/jssc.200800461. PMID: 19072899.
- Vermeij TA, Edelbroek PM. Simultaneous high-performance liquid chromatographic analysis of pregabalin, gabapentin and vigabatrin in human serum by precolumn derivatization with o-phtaldialdehyde and fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Oct 25;810(2):297-303. doi: 10.1016/j.jchromb.2004.08.018. PMID: 15380728.
- Pavlova V, Petrovska-Jovanovic S. Simultaneous determination of amphetamine, methamphetamine and caffeine in seized tablets by high-performance liquid chromatography.ActaChromatogr. 2007;18:157-167
- Wen J, Zhang WT, Cao WQ, Li J, Gao FY, Yang N, Fan GR. Enantioselective separation of mirtazapine and its metabolites by capillary electrophoresis with acetonitrile field-amplified sample stacking and its application. Molecules. 2014 Apr 17;19(4):4907-23. doi: 10.3390/molecules19044907. PMID: 24747648; PMCID: PMC6270698.
- Labat L, Dallet P, Kummer E, Dubost JP. Spectrophotometric, spectrofluorimetric, HPLC and CZE determination of mirtazapine in pharmaceutical tablets. J Pharm Biomed Anal. 2002; 15;28(2):365-71. doi: 10.1016/s0731-7085(01)00625-2.