More Information
Submitted: April 28, 2025 | Approved: May 09, 2025 | Published: May 12, 2025
How to cite this article: Sharma N, Kaushik R, Millo T, Behera C. Analyzing Maternal Inheritance of Mitochondrial DNA using PCR-RFLP. J Forensic Sci Res. 2025; 9(1): 054-058. Available from:
https://dx.doi.org/10.29328/journal.jfsr.1001081
DOI: 10.29328/journal.jfsr.1001081
Copyright License: © 2025 Sharma N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: mtDNA; PCR-RFLP; Maternal inheritance; Hypervariable region; Forensic genetics
Analyzing Maternal Inheritance of Mitochondrial DNA using PCR-RFLP
Nidhi Sharma1*, Ruchika Kaushik2, Tabin Millo3 and Chittaranjan Behera3
1Scientist, Department of Forensic Medicine, All India Institute of Medical Sciences, New Delhi, India
2Project Research Scientist, Department of Forensic Medicine, All India Institute of Medical Sciences, New Delhi, India
3Professor, Department of Forensic Medicine, All India Institute of Medical Sciences, New Delhi, India
*Address for Correspondence: Nidhi Sharma, Scientist, Department of Forensic Medicine, All India Institute of Medical Sciences, New Delhi, India, Email: [email protected]
Background & objectives: Mitochondrial DNA (mtDNA) contains valuable genetic information and plays a crucial role in missing person investigations, mass disasters, and forensic cases involving limited or degraded biological material. mtDNA is maternally inherited, with a highly variable control region divided into three hypervariable regions are generally used for forensic investigation. This study aimed to evaluate maternal inheritance patterns of mtDNA using PCR-RFLP techniques to confirm maternal relatedness.
Method: The study was designed after prior permission from the institute’s ethical committee in which subjects were enrolled. This pilot study analyzed 50 voluntary participants (mother-child pairs). DNA was extracted from blood or saliva, and the mtDNA hypervariable region (HV region) was amplified by PCR using specific primers for the HV1 region. The amplified fragments (1024 bp) were subjected to RFLP analysis using seven restriction endonucleases (Alu I, BsuR I (Hae III), Hinf I, HsYF31 (Dde I), Mbo I, Rsa I, and SsPI) to reveal morphotypes.
Results: The study identified five morphotypes for Alu I, three for BsuR I (Hae III) and Rsa I, two for Hinf I, and one each for HsYF31 (Dde I), Mbo I, and SsPI. There was minimal genetic polymorphism in the hypervariable region among unrelated individuals, but consistent restriction patterns were observed between mothers and their children in same pair.
Conclusion: The findings demonstrate the low genetic polymorphism in the hypervariable region among unrelated individuals and consistent restriction patterns within maternal pairs, underscoring mtDNA's utility in forensic and genealogical applications.
Human mitochondrial DNA (mtDNA) is a pivotal tool in evolutionary studies, forensic identification, and diversity analysis [1]. In the cytoplasm of nucleated cells, mtDNA is maternally inherited, with its hypervariable region, being one of the most variable regions. This variability facilitates individual differentiation in forensic investigations, particularly with degraded samples. Polymerase chain reaction - restriction fragment length polymorphism (PCR-RFLP) analysis provides a robust method for studying mtDNA polymorphisms to relate or discriminate the species [2]. The first step in a RFLP analysis is the Polymerase Chain Reaction (PCR) that is; amplification of a fragment containing the variation. This is followed by treatment of the amplified fragment with an appropriate restriction enzyme to produce different polymorphic fragment and used as markers for identification of species. Since the presence or absence of the restriction enzyme recognition site results in the formation of restriction fragments of different sizes, allele identification can be done by electrophoretic method in which fragments resolve as per size [3].
Mitochondria are subcellular organelles containing an extra-nuclear circular genome other than nuclear genome. Mitochondria often described as the powerhouse of the cell, a remnant of their evolutionary origin as endosymbiotic organisms in each living cell (all animal and plant cells). Because mtDNA is transmitted solely through the maternal line, it serves as a reliable marker for tracing maternal ancestry and establishing maternal relatedness. The hypervariable regions within the D-loop are particularly useful for genetic analysis due to their higher mutation rates compared to other genomic regions [4]. These features make mtDNA an indispensable tool in forensic genetics, especially when nuclear DNA is unavailable or degraded [5].
Previously, only a few studies have been conducted, which demonstrated the utility of mtDNA in solving historical cases, tracing human migrations, and identifying victims of mass disasters [6]. The hypervariable regions (HV1 and HV2) of human mtDNA suggest a shared maternal lineage originating from a common founder, followed by subsequent maternal gene flow within Indian population groups [7]. This study seeks to contribute to this eminent field by investigating mtDNA polymorphisms in maternally related individuals and evaluating their consistency through RFLP techniques.
Mitochondrial DNA can be characterized by the high rate of mutation and polymorphism. It is reported that an individual's mother, siblings, and other maternally related family members have identical mtDNA polymorphism at the hypervariable region. However, to the best of our knowledge, only a few studies have examined lineage determination using the RFLP technique in mtDNA, and those were also very old, dating back to the 1990s. Though PCR-RFLP is considered as an old technique, its robustness has kept this technique reliable in some instances. Therefore, the present study aimed to understand the maternal inheritance pattern of mitochondrial DNA in unrelated individuals by the PCR-RFLP technique to assess polymorphic sites present in the HV1 region of paired samples (mother-child). We have evaluated the discriminatory power through this technique using various restriction enzymes, allowing for the differentiation of individuals based on restriction profiles.
This study was conducted in the Department of Forensic Medicine at AIIMS, New Delhi, India. A total of 50 mother-child pairs volunteered to participate. Informed consent was obtained, and the study was approved by the institute's ethics committee. Blood or saliva samples were collected from participants. DNA was extracted using organic methods (PCI), a Qiagen mini blood kit, and the Xpress DNA Saliva Kit (Mag Genome Technologies Pvt. Ltd) as per instruction. The quality and quantity of DNA yielding was assessed by using agarose gel electrophoresis and the OD value of the sample by using a spectrophotometer (Thermo Fischer Nanodrop1000). The DNA purity was assessed by measuring the absorbance ratio of DNA samples at 260 nm and 280 nm, if absorbance quotient (Q) is 1.7 to 2 the DNA were considered for further evaluation.
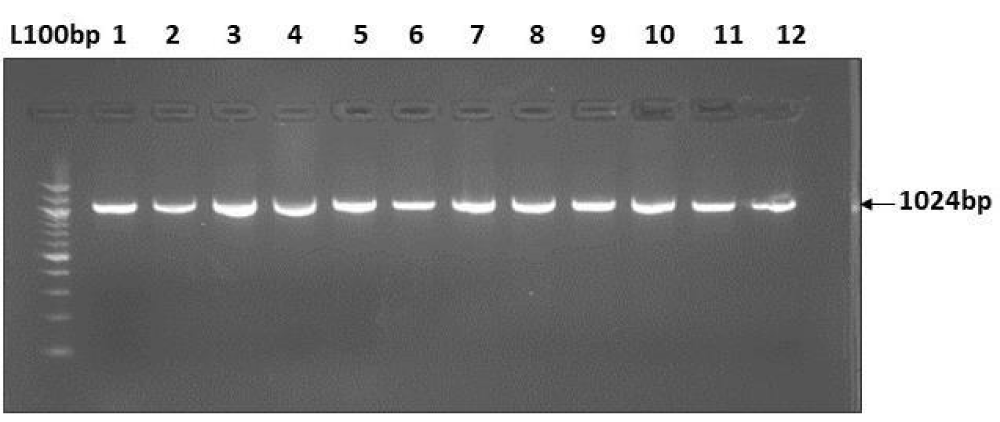
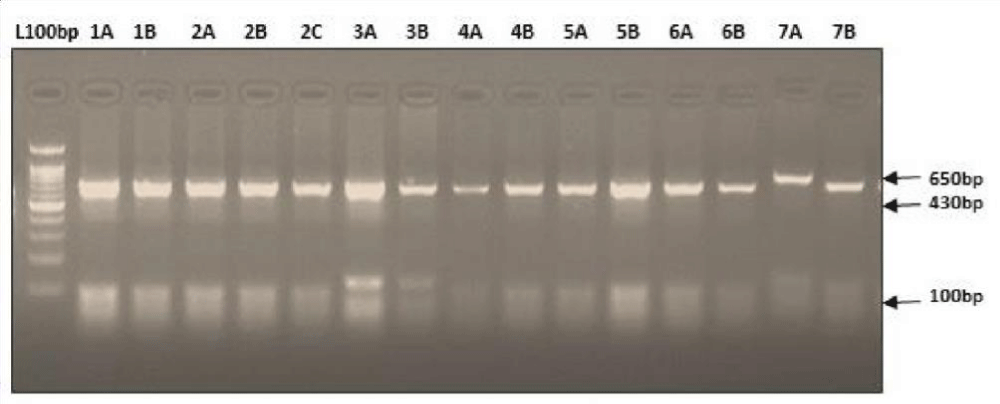
PCR amplification and RFLP of the mtDNA hypervariable region (HV1) targeted this region of interest present in the hypervariable region of the mtDNA. PCR amplifications were performed under standard reaction conditions by using published primers. Each reaction tube contained 2x PCR master mix (Thermo Fisher scientific) which includes reaction buffer, 4 mM MgCl2, 0.4 mM of each dNTPs, Taq DNA polymerase (0.05 U/µl) and each primer at a concentration of 20 pmol (synthesized by Eurofins Genomics India Pvt. Ltd) Mt-F1:5’CACCATTAGCACCCAAAGCT-3’,Mt-R1:5’-CTGTTAAAAGTGCATACCGCCA-3’ (HV1- for amplicons size 1024bp) [8]; and 2 µL of DNA. PCR was performed with the following conditions: 3 min at 95 °C followed by 40 cycles consisting 30 sec. of denaturation at 95 °C, 30 sec. of annealing at 560 and 40 sec of extension at 72 0C after 40 cycle extension was continued for an additional 10 min. For detection, 2 µL of the PCR product were electrophoresed on 2% agarose gel stained with ethidium bromide (Figure 1).
Figure 1: PCR amplification of 1024 bp HV1 region of mtDNA.
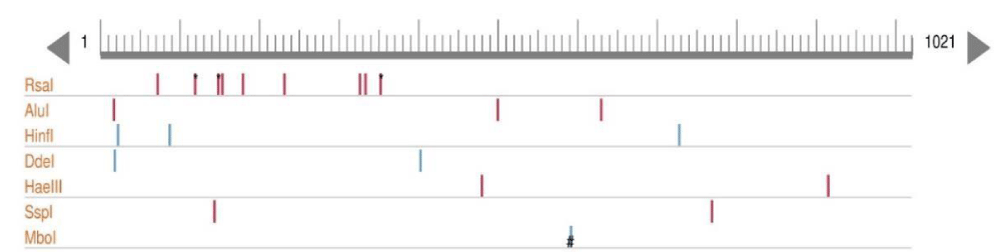
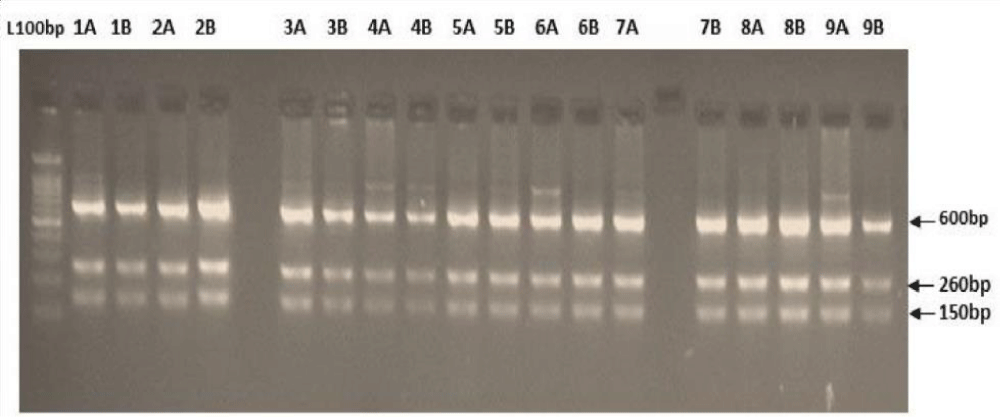
PCR-RFLP techniques generally analyze the frequency of DNA digestion by restriction enzymes which depends on their recognition sequences. The shorter the recognition sequences, the higher the cutting frequency. Selected restriction enzymes have recognition sequences of 4, 6, or 8 base pairs. The seven restriction enzymes: Alu I, BsuR I (Hae III), Hinf I, HsYF31 (Dde I), Mbo I, Rsa I, and SsPI were used have 1 to 8 cutting sites (Figure 2). PCR amplicons were digested with seven restriction enzymes: Alu I, BsuR I (Hae III), Hinf I, HsYF31 (Dde I), Mbo I, Rsa I, and SsPI. Digestion reactions (20 μl) contained 10 μl of PCR product, 2 μl of buffer, 0.2 μl of enzyme, and 7.8 μl of distilled water. Samples were incubated at 37 °C overnight to ensure complete digestion at recognition sites. Digested fragments were separated on 2% agarose gels where the fragments are separated (according to size) and compared to a 100 bp ladder.
Figure 2: Pictorial view of the enzymes cutting sites.
A total of 50 mother-child pairs were recruited for this study. DNA was successfully isolated from all samples, the yield of the extracted DNA from the samples was evaluated using a spectrophotometer and the gel electrophoresis. DNA extraction from blood samples resulted in 1777 ng/µL and in saliva 32 - 72 ng/µl, the purity of the extracted DNA from the blood (1.7 - 1.8) and saliva samples (1.4 - 1.5). The gel photo shows a representative amplification of hyper variable regions using the primers mentioned in the methods. The size markers demonstrate that all amplicons are of the expected size. (Figure 1) and subsequently processed for RFLP analysis. After screening on the 2% agarose gel, morph types (patterns) were determined for each enzyme used (Table 1).
| Table 1: Restriction morphs for MtDNA D-loop region and their frequencies (1AB - 50AB). | ||||
| Restriction enzymes | Fragment length (bp) | No. of samples N = 100 | Morphotypes (patterns) |
|
| 1 | AluI | 500,400,100 | 48 (24 pairs) | 5 |
| 500,400,110 | 38 (19 pairs) | |||
| 400,300,290,100 | 2 (1 pair) | |||
| 400,300,290 | 2(1 pair) | |||
| 400,300,100 | 2(1 pair) | |||
| 2 | BsuRI | 550,400,100 | 78 (39 pairs) | 3 |
| 500,100 | 10 (5 pairs) | |||
| 500,490,100 | 4 (2 pairs) | |||
| 3 | Hinf1 | 700, 300 | 90 (45 pairs) | 2 |
| 700,500,300,200 | 2(1pair) | |||
| 4 | HsyF31(Dde) | 600,390 | 100 (50 pairs) | 1 |
| 5 | Mbo1 | 600, 430 | 100 (50 pairs) | 1 |
| 6 | Rsa1 |
650, 100 | 84(42 pairs) | 3 |
| 650,150, 100 | 2 (1 pair) | |||
| 650,430, 100 | 6 (3 pairs) | |||
| 7 | Ssp1 | 600, 260,150 | 100 (50 pairs) | 1 |
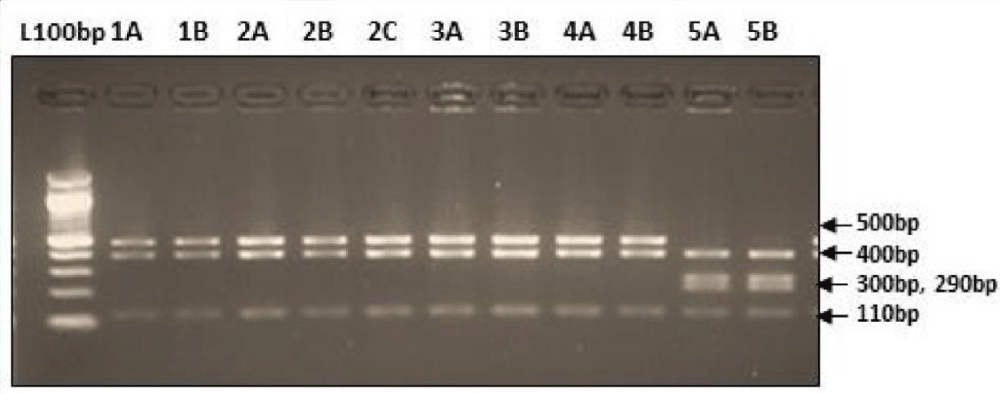
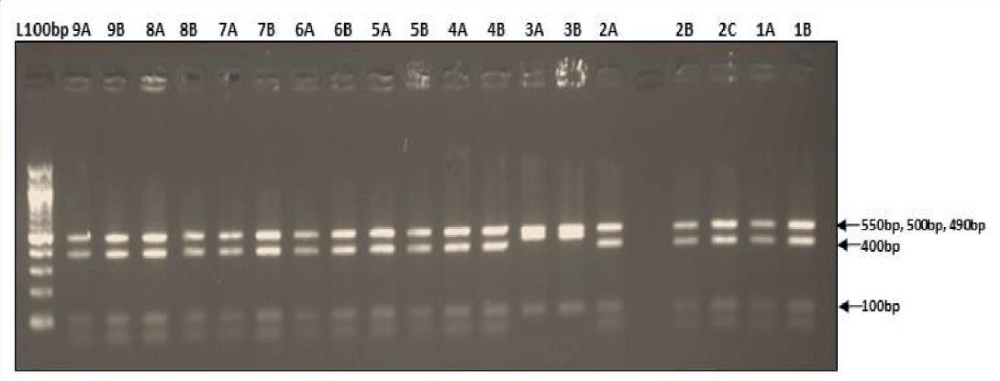
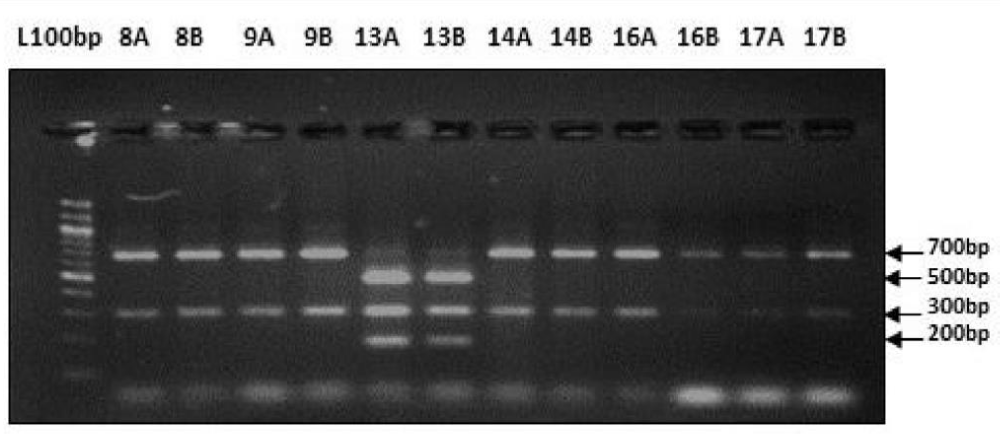
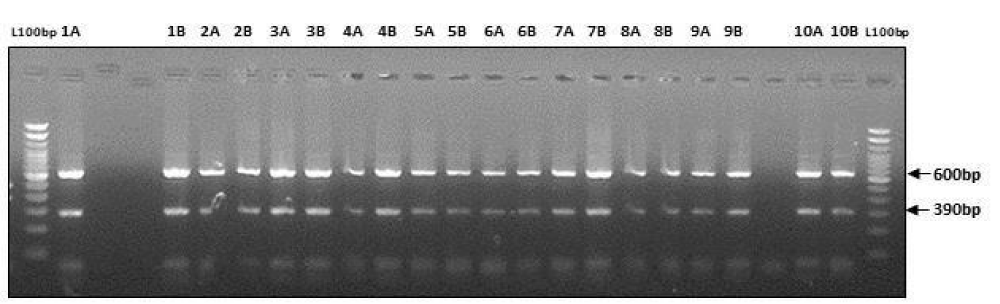
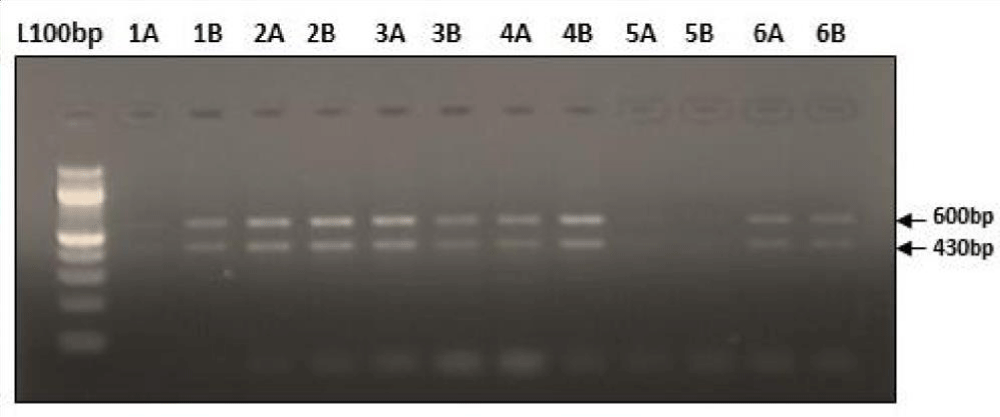
Digestion of amplified fragments of mtDNA hypervariable region by restriction endonuclease. The restriction enzymes, Alu I, BsuR1 (Hae III), Hinf I, HsYF31(Dde I) Mbo 1, Rsa I, Ssp1, presented polymorphism in Figures 3-9. MtDNA PCR-RFLP techniques were used to reveal polymorphism of mtDNA hypervariable region. PCR product for mtDNA hypervariable region on agarose gel showed an amplified fragment of about 1024bp (Figure 1), which is digested using enzymes (PCR- RFLP techniques generally used for frequencies of restriction enzymes digesting DNA).
Figure 3: Restriction digestion pattern using AluI enzyme.
Figure 4: Showing digestion of Restriction enzyme BsuR1 fragment size.
Figure 5: Showing digestion of Restriction enzyme Hinf1 fragment size.
Figure 6: Showing digestion of Restriction enzyme HsYF31 (Dde1) fragment size.
Figure 7: Showing digestion of Restriction enzyme Mbo1 fragment size.
Figure 8: Showing digestion of Restriction enzyme Rsa1fragment size.
Figure 9: Showing digestion of Restriction enzyme Ssp1 fragment size.
Polymerase Chain Reaction-restriction fragment length polymorphism (PCR-RFLP) based analysis is a popular technique for genotyping. This technique used to find and differentiate the Single Nucleotide Polymorphisms (SNPs), Multiple Nucleotide Polymorphisms (MNPs) and insertion /deletion of bases that sometime associated with creation or nullify of a restriction enzyme recognition site [9]. The findings of present study highlight the low genetic polymorphism of the mtDNA D-loop region among unrelated individuals while confirming consistent patterns within maternal pairs. These results align with prior research by Doosti, et al. which also demonstrated limited variability in the D-loop region within maternally related populations [10]. Such consistency underscores the reliability of mtDNA analysis for forensic and genealogical applications. The evolutionary stability of the hypervariable region has significant implications. While it is highly variable within a population, it remains conserved among closely related individuals, providing a robust marker for maternal lineage. Various studies on ethnicity conducted in the European peninsula further validate this phenomenon, demonstrating its utility in tracing maternal ancestry across diverse populations [11-13].
However, this study also raises questions about the variability of mtDNA in different populations. Research by Doosti, et al. [10] revealed higher levels of polymorphism in the Bakhtiarian population compared to our findings. These differences may be attributed to genetic diversity, environmental factors, or methodological variations. This highlights the need for population-specific studies to better understand mtDNA variability. The practical applications of mtDNA analysis extends beyond forensics. It plays a critical role in historical, anthropological, and medical genetics [14]. For example, understanding disease-associated mtDNA mutations provides insights into the maternal inheritance of certain conditions.
While PCR-RFLP remains a cost-effective and accessible technique for mitochondrial DNA analysis, particularly in resource-limited settings, it is important to acknowledge its limitations in discriminatory power [9]. The method is constrained by its reliance on restriction enzyme recognition sites, which may not capture the full extent of sequence variability, especially in highly polymorphic regions. In contrast, emerging techniques such as Next-Generation Sequencing (NGS) offer much higher resolution by enabling the analysis of complete mitochondrial genomes, thereby allowing the detection of even minor variants and heteroplasmic mutations [15].
To enhance the robustness of forensic investigations, a complementary approach can be adopted wherein PCR-RFLP serves as an initial screening tool, followed by targeted NGS for samples requiring higher discriminatory resolution. Such integration not only improves accuracy but also maintains cost-efficiency. Incorporating modern platforms with traditional techniques aligns well with the evolving standards of forensic genomics and offers a scalable solution for diverse casework scenarios [15].
Another limitation of this study is that we did not include comparative analysis across distinct maternal lineages or ethnic groups due to less sample size of the study. Though they were nonblood relative and belong to different states but they cannot represent the large population of even that particulatr state in India. This remains an important direction for future research to evaluate mitochondrial polymorphism patterns across diverse population.
India's extensive genetic diversity, shaped by its complex population structure, can influence the interpretation of mtDNA variations detected by RFLP. Since certain polymorphisms may be population-specific, mtDNA profiling using RFLP must be validated across diverse subgroups to ensure forensic reliability and broader applicability. mtDNA-RFLP proves particularly valuable in forensic cases involving degraded samples, such as in mass disasters or old skeletal remains, where nuclear DNA is often unusable. For instance, it can help identify victims of a plane crash through maternal lineage or link aged bone fragments to missing persons via maternal relatives.
Future research should focus on expanding the sample size and including individuals from diverse ethnic backgrounds. Additionally, integrating advanced sequencing technologies with traditional RFLP methods could provide a more comprehensive understanding of mtDNA variability and its applications.
This study highlights the reliability of PCR-RFLP analysis of mtDNA hypervariable regions in assessing maternal inheritance. Although polymorphism levels were low, the consistent patterns observed between mothers and offspring underscore the forensic and genealogical significance of mtDNA. Fragment pattern analysis reveals genetic polymorphisms useful for identification of specific alleles or genetic markers. However, the limited discriminatory power of the RFLP technique suggests that advanced methods such as PCR-based assays and next-generation sequencing (NGS), and other emerging technologies, offer greater accuracy and resolution. Future research should focus on analysing diverse populations and exploring additional mtDNA regions to enhance the robustness of maternal lineage studies.
- Butler JM, Levin BC. Forensic applications of mitochondrial DNA. Trends Biotechnol. 1998;16(4):158–62. Available from: https://doi.org/10.1016/s0167-7799(98)01173-1
- Bermisheva MA, Viktorova TV, Khusnutdinova EK. Polymorphism of Human Mitochondrial DNA. Russ J Genet. 2003;39(8):849–59. Available from: https://doi.org/10.1023/A:1025319702808
- Marwal A, Sahu AK, Gaur RK. Chapter 16 - Molecular Markers: Tool for Genetic Analysis. In: Verma AS, Singh A, editors. Animal Biotechnology. Academic Press. 2014;289–305. Available from: https://doi.org/10.1016/B978-0-12-416002-6.00016-X
- Wallace DC, Brown MD, Lott MT. Mitochondrial DNA variation in human evolution and disease. Gene. 1999;238(1):211–30. Available from: https://doi.org/10.1016/s0378-1119(99)00295-4
- Case JT, Wallace DC. Maternal inheritance of mitochondrial DNA polymorphisms in cultured human fibroblasts. Somatic Cell Genet. 1981;7(1):103–8. Available from: https://doi.org/10.1007/BF01544751
- Merheb M, Matar R, Hodeify R, Siddiqui SS, Vazhappilly CG, Marton J, et al. Mitochondrial DNA, a Powerful Tool to Decipher Ancient Human Civilization from Domestication to Music, and to Uncover Historical Murder Cases. Cells. 2019;8(5):433. Available from: https://doi.org/10.3390/cells8050433
- Sharma S, Saha A, Rai E, Bhat A, Bamezai R. Human mtDNA hypervariable regions, HVR I and II, hint at deep common maternal founder and subsequent maternal gene flow in Indian population groups. J Hum Genet. 2005;50(10):497–506. Available from: https://doi.org/10.1007/s10038-005-0284-2
- Salas A, Lareu MV, Carracedo A. Heteroplasmy in mtDNA and the weight of evidence in forensic mtDNA analysis: A case report. Int J Legal Med. 2001;114(3):186–90. Available from: https://doi.org/10.1007/s004140000164
- Parson W, Bandelt HJ. Extended guidelines for mtDNA typing of population data in forensic science. Forensic Sci Int Genet. 2007;1(1):13–9. Available from: https://doi.org/10.1016/j.fsigen.2006.11.003
- Doosti A, Dehkordi P. Genetic Polymorphisms of Mitochondrial Genome D-loop Region in Bakhtiarian Population by PCR-RFLP. Int J Biol. 2011;3(4):41. Available from: https://doi.org/10.5539/ijb.v3n4p41
- Bandelt HJ, Forster P, Sykes BC, Richards MB. Mitochondrial portraits of human populations using median networks. Genetics. 1995;141(2):743–53. Available from: https://doi.org/10.1093/genetics/141.2.743
- Richards M, Macaulay V, Hickey E, Vega E, Sykes B, Guida V, et al. Tracing European founder lineages in the Near Eastern mtDNA pool. Am J Hum Genet. 2000;67(5):1251–76. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC1288566/
- Torroni A, Bandelt HJ, D’Urbano L, Lahermo P, Moral P, Sellitto D, et al. mtDNA analysis reveals a major late Paleolithic population expansion from southwestern to northeastern Europe. Am J Hum Genet. 1998;62(5):1137–52. Available from: https://doi.org/10.1086/301822
- Syndercombe Court D. Mitochondrial DNA in forensic use. Emerg Top Life Sci. 2021;5(3):415–26. Available from: https://doi.org/10.1042/ETLS20210204
- Butler JM. Advanced Topics in Forensic DNA Typing: Interpretation. Elsevier; 2014. Available from: https://shop.elsevier.com/books/advanced-topics-in-forensic-dna-typing-interpretation/butler/978-0-12-405213-0