More Information
Submitted: August 23, 2023 | Approved: August 26, 2023 | Published: August 28 2023
How to cite this article: Kearse KP. The Effect of Humidity on Blood Serum Pattern Formation and Blood Transfer. J Forensic Sci Res. 2023; 7: 040-048.
DOI: 10.29328/journal.jfsr.1001048
Copyright License: © 2023 Kearse KP. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Blood; Plasma blister; Serum; Humidity; Transfer
The Effect of Humidity on Blood Serum Pattern Formation and Blood Transfer
Kelly P Kearse*
Knoxville Catholic High School, Knoxville, TN, USA
*Address for Correspondence: Kelly P Kearse, Knoxville Catholic High School, Knoxville, TN, USA, Email: [email protected]
A detailed knowledge of the drying properties of blood is important for a more complete understanding of the forensic information that may exist at a crime location. Although the effect of relative humidity on the general properties of blood drying has been evaluated, relatively little information exists regarding the alterations of blood serum distribution that may occur during the drying process. Moreover, the influence of humidity on the ability of dried blood drops to transfer from skin to absorbent material has never been studied. The data in the current report show that blood serum pattern formation is distinctly altered by increased humidity in drying drops of blood. In addition, these data document that high humidity conditions were sufficient to remoisten dried blood drops such that they were able to transfer to the absorbent material, with the original bloodstain pattern maintained.
Numerous studies exist on the drying properties of blood and related fluids on various surfaces and the influence of variables such as temperature and humidity on this process [1-37]. However, relatively little information is known about the distribution of plasma in drying blood drops under varying conditions as its presence is typically masked by red blood cells in solutions of whole blood. Recent studies have demonstrated that ultraviolet-365 (UV-365) is an effective alternative light source (ALS) for the detection of blood serum in dried stains [38,39], which was used to monitor plasma pattern formation in drying drops of blood on both glass and skin [40]. These studies demonstrated that plasma migration is an active process in the initial stages of blood drying, with the initial and rapid formation of a central “plasma blister”, which subsequently lessens and is accompanied by the thickening of the plasma ring at the periphery [40].
The shape and distribution of dried bloodstains on the skin or clothing of the victim is a function of the circumstances under which the crime was committed. In situations where the body may have been moved, blood transfer is possible, which could provide valuable clues in recreating exactly what may have happened. Typically, once blood is dried, the possibility of transference is greatly reduced as a moist, gelatinous-like state is necessary for blood to be effectively absorbed [6]. Studies have shown that increased humidity resulted in delayed drying times of fresh blood [5]; however, little to no information exists about the effect of humidity on the dried state, especially the ability to confer transfer properties from the skin. Remoistening of dried blood could provide a means by which blood transfer may occur hours (or even days) after the initial crime.
Here, the effect of humidity on blood plasma and serum pattern formation in drying blood was examined at varying temperatures. In addition, the remoistening of dried blood and transfer ability were evaluated.
Blood, murine skin, porcine skin and material
Human blood was obtained by the finger stick method using a Health Lancing device (CVS Pharmacy, USA) fitted with a micro lancet (CVS Pharmacy, USA). Human blood serum was obtained from Innovative Research (Innovative Research Company, Novi, MI), and purchased as pooled human serum of the clot. Blood was dropped onto Parafilm® M Laboratory Film (Bemis Company, Inc., Oshkosh, WI) and approximately 10-50 microliters were transferred to the drying substrate. Newborn mice approximately 1-2 weeks of age were obtained from Rodent Pro LLC (Inglefield, IN). Porcine skin (with fat removed) was obtained from Animal Technologies (Tyler, TX), a leading supplier of skin utilized in medical schools for human surgery practice. For blood transfer experiments, Whatman® 1 mm filter paper (Whatman, Maidstone, Kent, UK), cotton (a white t-shirt), or natural linen (Rawganique, Blaine, WA) was used. Glass microscope slides (BS-72P-100S-22) were obtained from AmScope (Irvine, CA).
Sample preparation, humidity chambers, incubation, and rainwater rinsing
For experiments involving both fresh and dried blood, blood was added to glass slides or murine skin and allowed to dry for 3-4 hours. Fresh blood was then added to glass slides or murine skin and both sets (fresh, and dried) were placed under the appropriate conditions. Humidity chambers were created using a black plastic square box (21.5 cm x 21.5 cm x 4.5 cm) with a locking lid, obtained from a local restaurant. A paper towel was folded in four, placed in the upper region of the box, and saturated with water. All excess water was removed before the samples were added. Humidity was measured using an Antoki (Shanghai, China) humidity gauge placed inside the container which gave a value of approximately 88% - 91% in this system, referred to as high humidity. For low humidity samples (room temperature humidity), the setup was the same, except that the saturated paper towel was omitted, giving a humidity value of approximately 41% - 42%, similar to that measured in the room. Samples were placed inside a 25 L incubator (Happybuy Store, San Paulo, Brazil) set at the desired temperature for 12-15 hours. The following settings were used: low temperature (13 oC), room temperature (22 oC), and high temperature (33 oC). Multiple incubators were used in these experiments and samples under different conditions were run in parallel.
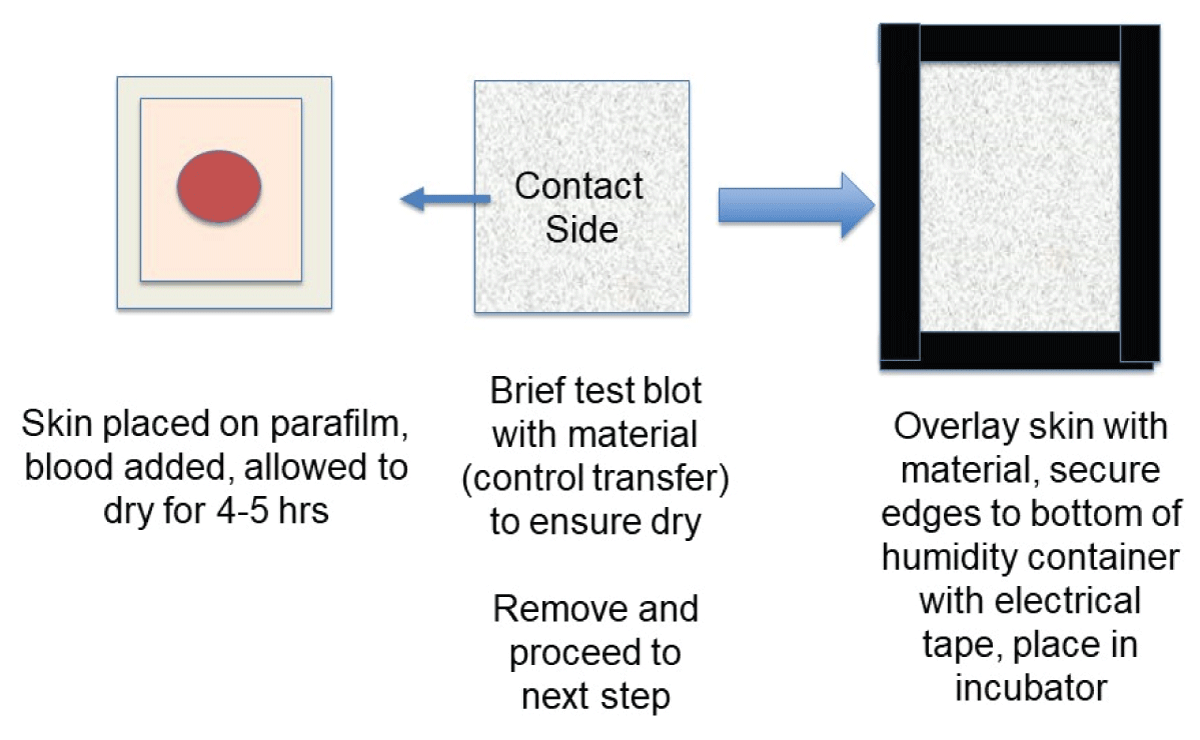
For overlay experiments, an approximately 4 cm x 4 cm piece of porcine skin was placed on a piece of parafilm, blood added, and allowed to dry for 4-5 hours. To ensure that the blood was sufficiently dry such that no transfer initially occurred, a test blot (control transfer) was performed each time with a separate piece of material. The test material was then removed and the skin overlayed with a piece of cotton or linen. Electrical tape was used to secure the edges of the material to the humidity container and samples were placed inside of the incubator. At the end of the incubation period, the material was removed and photographed. Rainwater was collected and added to a 50 ml fine mist mini spray bottle. The bottle was held approximately 10 inches away from the subject and depressed 15-20 times. Samples were allowed to dry at room temperature and photographed.
Ultraviolet light sources and photography
An LED UV flashlight, LED-UV301-365 nm (Shenzhen Lightfe Light Limited, Shenzhen, China), positioned at a ~ 45o angle from the subject was used as the UV source. Photographs were taken with either a Sony 6500 digital camera fitted to a Unitron ZST stereomicroscope or a handheld Sony RX100 digital camera.
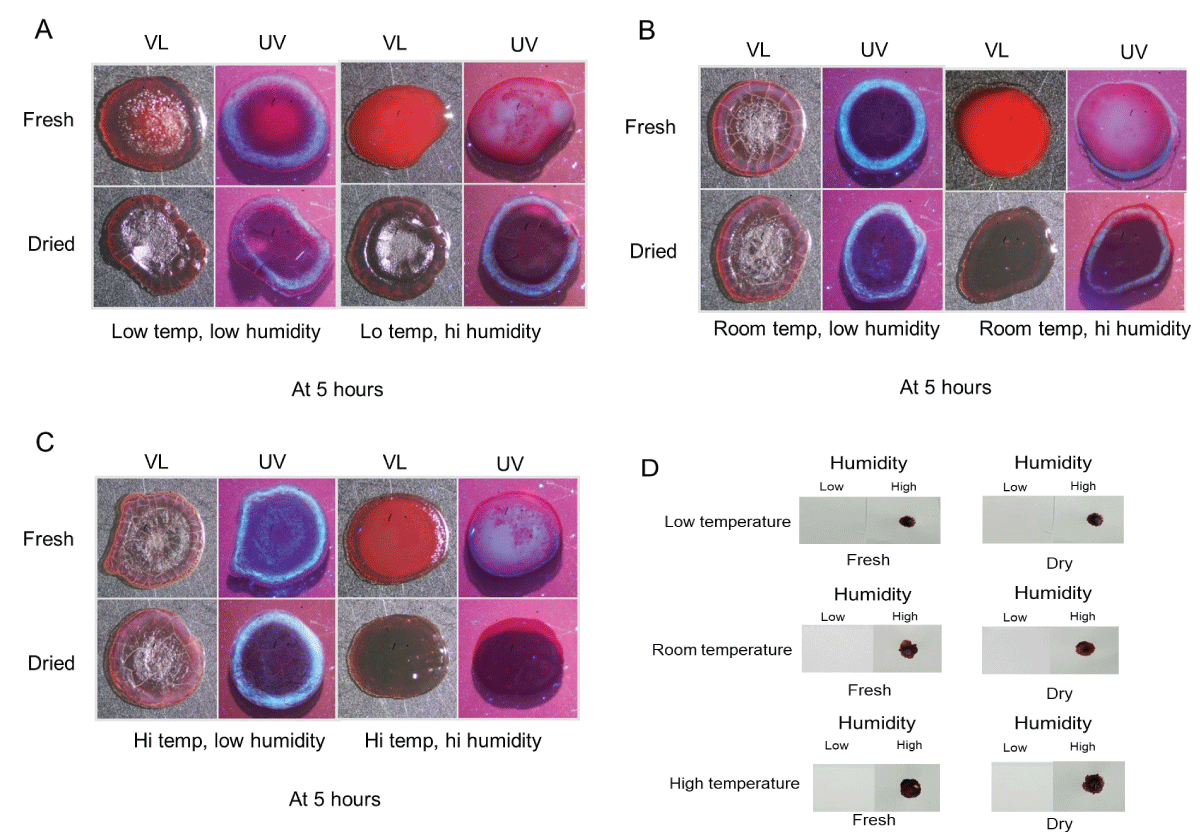
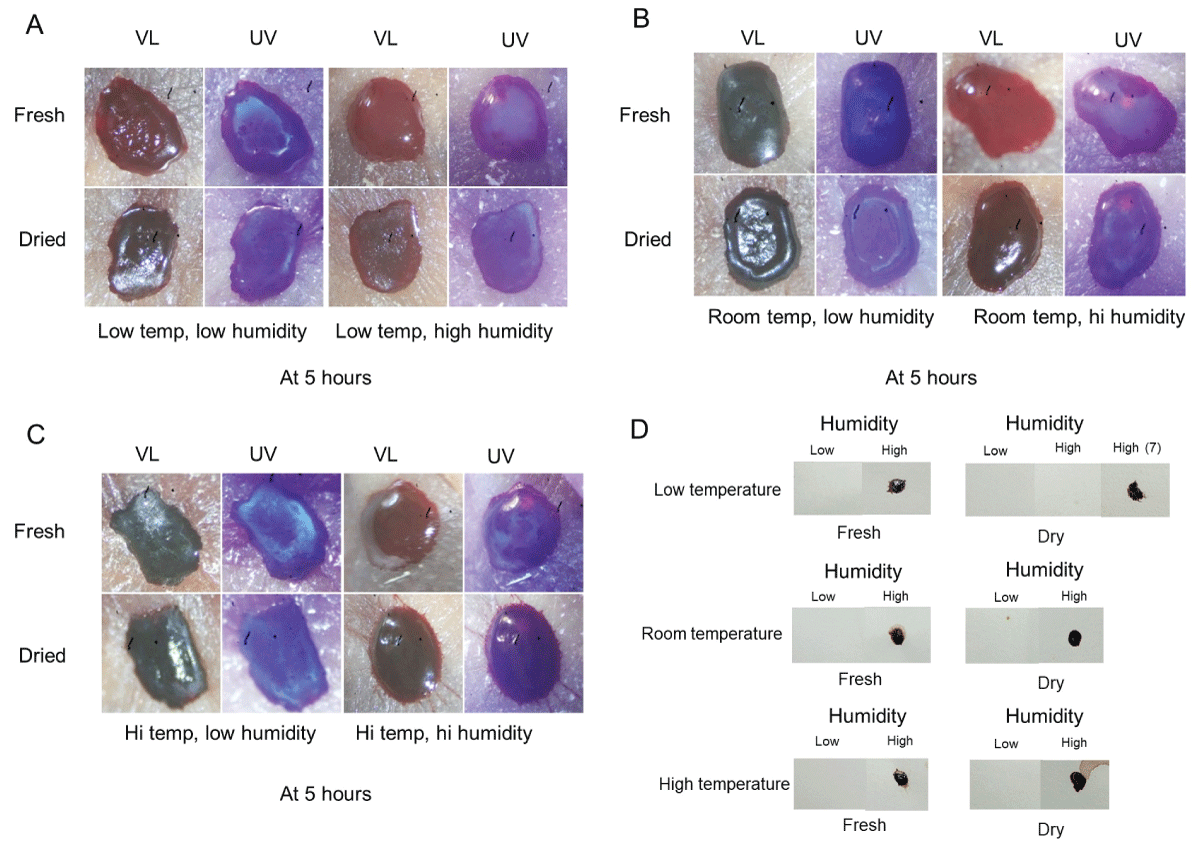
While substantial information exists regarding the general drying properties of whole blood, the pattern formation of plasma and serum has been visualized only recently [40]. In general, the initial stages involve the development of a plasma blister in the center which subsequently diminishes and is followed by the creation of a fluorescent serum halo at the edges; the plasma blister and serum halo are effectively visualized under ultraviolet light. The goal of these initial experiments was to determine the effects of temperature and humidity on plasma morphology in drying and dried blood and to evaluate the effect of humidity on blood transfer. Fresh or dried blood drops on glass slides were incubated for five hours at low, room, or high temperatures under conditions of low or high humidity (see Materials and Methods for specific conditions). Samples were then removed and immediately photographed under normal visible light (VL) or ultraviolet light (UV). As shown in Figure 1, the appearance of fresh blood at low temperature and low humidity was slightly different from low humidity groups at room or high temperatures, with a slightly less developed central portion (Figures 1A-C, upper left), although ultraviolet light revealed that a well-defined fluorescent serum halo had developed around the corona in all three groups (Figures 1A-C, upper left) [40]. Similar results were seen for dried blood incubated under low humidity at varying temperatures (Figure 1A-C, bottom left). Neither fresh nor dried blood samples incubated under low humidity conditions were sufficiently fluid or tacky to transfer to filter paper (Figure 1D). The appearance of fresh blood incubated under high humidity was markedly different than low humidity groups, with plasma blisters visible under ultraviolet at all three temperatures (Figures 1A-C, upper right) [40]. Relatedly, fresh blood incubated in high-humidity conditions was able to imprint filter paper in all three temperature groups (Figure 1D).
Dried blood placed in high humidity had a gel-type appearance at room temperature and higher, and a fluorescent serum halo was not observed in high-temperature groups (Figures A-C, bottom right). Most notably, similarly to fresh blood, dried blood exposed to high humidity was sufficiently reliquefied to imprint filter paper (Figure 1D).
Figure 1: Plasma/serum pattern formation and transfer ability of blood incubated under various temperature and humidity conditions. (A-C): Blood was placed on glass slides, incubated for five hours under the indicated conditions, and immediately photographed under visible light (VL) and ultraviolet light (UV). See text for specific details. (D) Blood was cultured as above and placed in contact with a piece of filter paper to evaluate the transfer properties under the conditions used.
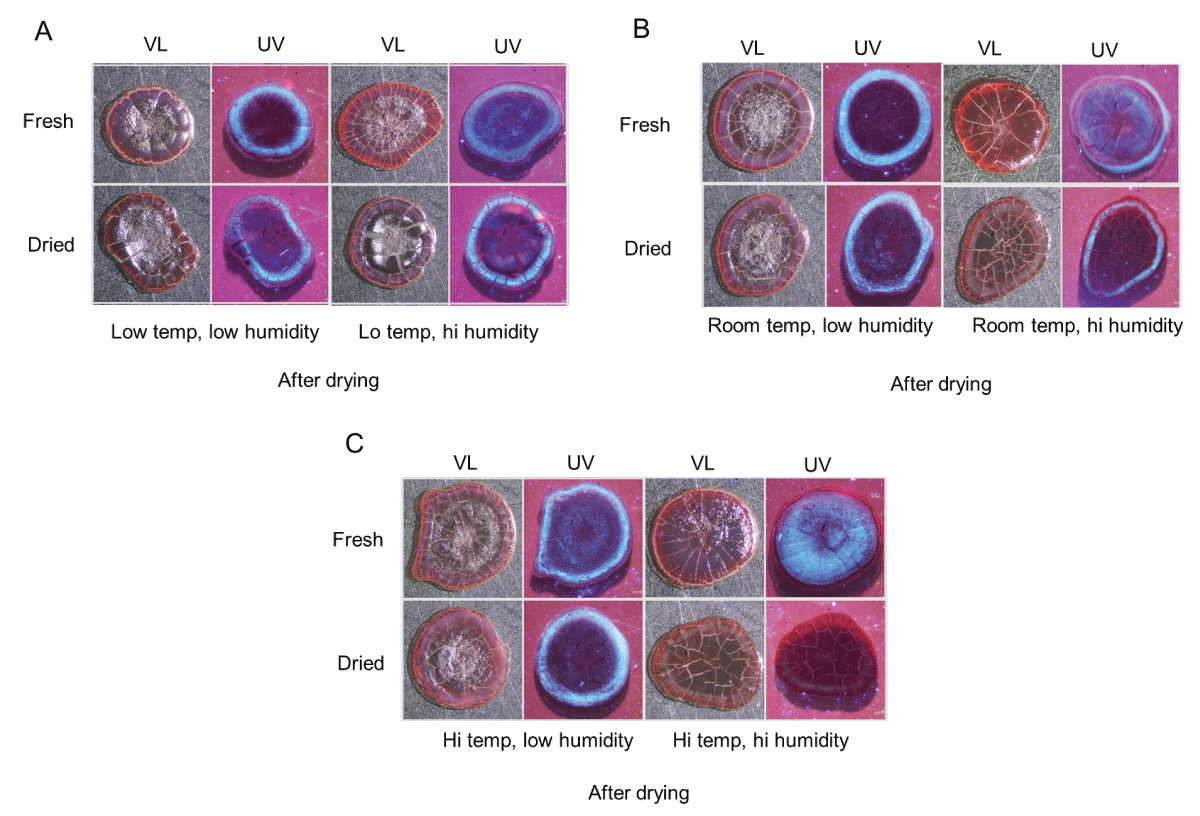
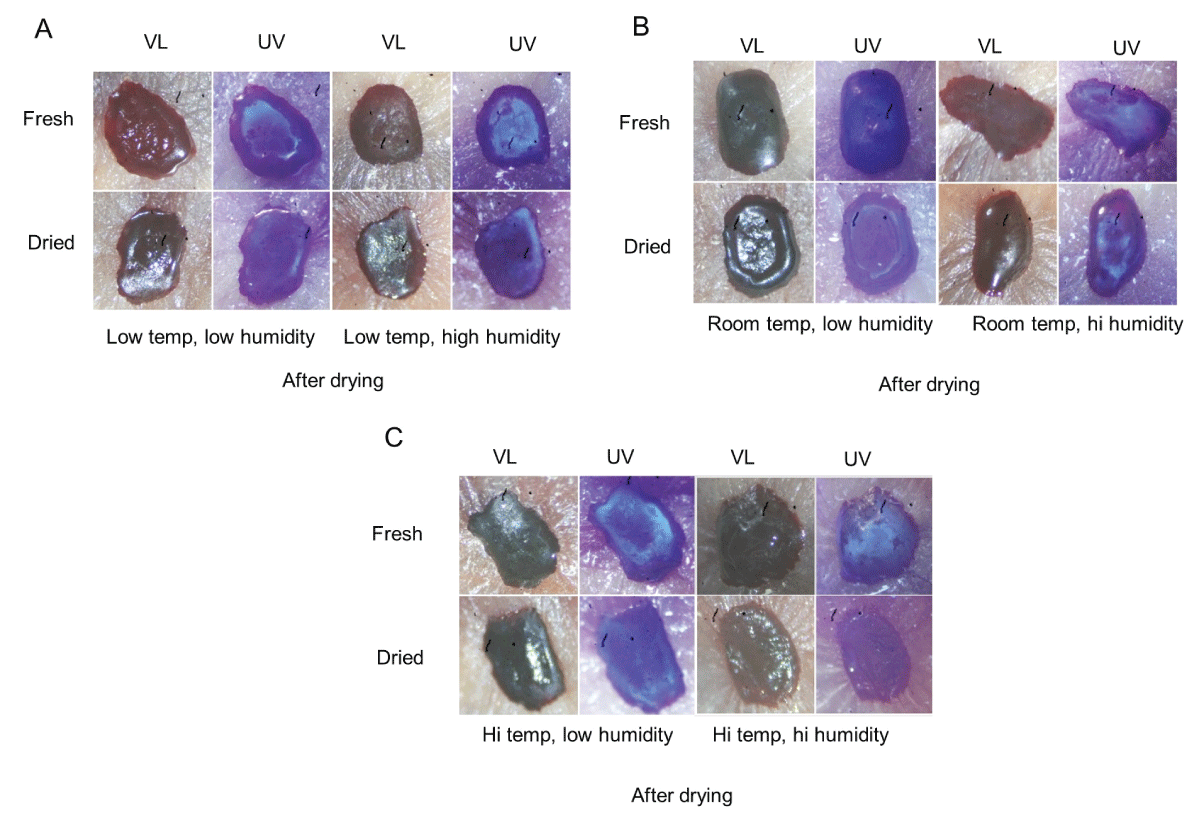
All the above samples were allowed to dry completely (at room temperature and low humidity) and then rephotographed to examine their final appearance (Figures 2A-C). Fresh blood that was exposed to high humidity showed a more “frosted” appearance at the center after drying, which was especially prominent at high temperatures (Figures 2A-C, upper right, UV). Like what was observed at five hours, serum halos were noticeably missing under conditions of high temperature and high humidity (Figure 2C, bottom right, UV).
Figure 2: Serum pattern formation of dried blood incubated under various temperature and humidity conditions. The samples in Figure 1A-C were allowed to dry at room temperature under low humidity and then rephotographed as above under visible light (VL) and ultraviolet light (UV). See text for specific details.
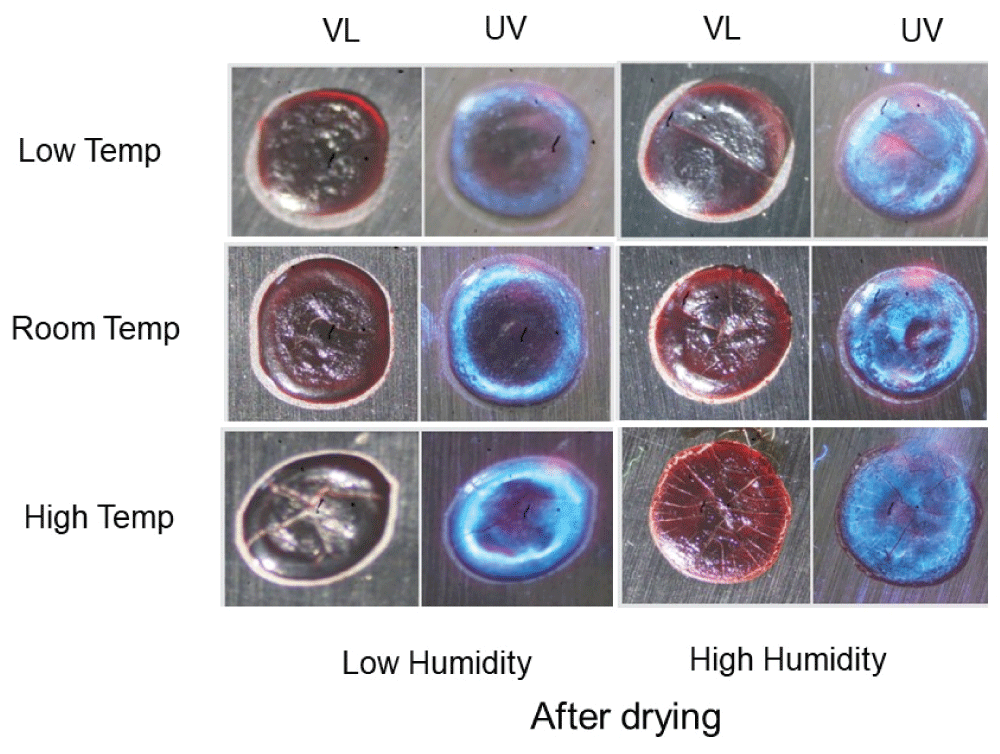
Similar experiments were performed with fresh blood added to a metal knife blade, yielding comparable results (Figure 3). Serum was located throughout the sample with a frosted appearance in high humidity groups (Figure 3, right-hand side, UV); and a distinct fluorescent serum halo was present in samples exposed to low humidity (Figure 3, left-hand side, UV).
Figure 3: Serum pattern formation of dried blood on a metal knife blade incubated under various temperature and humidity conditions. Fresh blood was added to a metal knife blade and incubated under the indicated temperature and humidity conditions for five hours. Samples were then allowed to dry at room temperature under low humidity conditions and photographed under visible light (VL) and ultraviolet light (UV). Note the frosted appearance under high humidity conditions, indicative of serum spreading throughout the bloodstain.
Taken together, these results collectively demonstrate plasma and serum pattern formation is altered by high humidity in drying drops of blood. Additionally, these data show that dried bloodstains were sufficiently moistened under high humidity conditions to transfer onto filter paper.
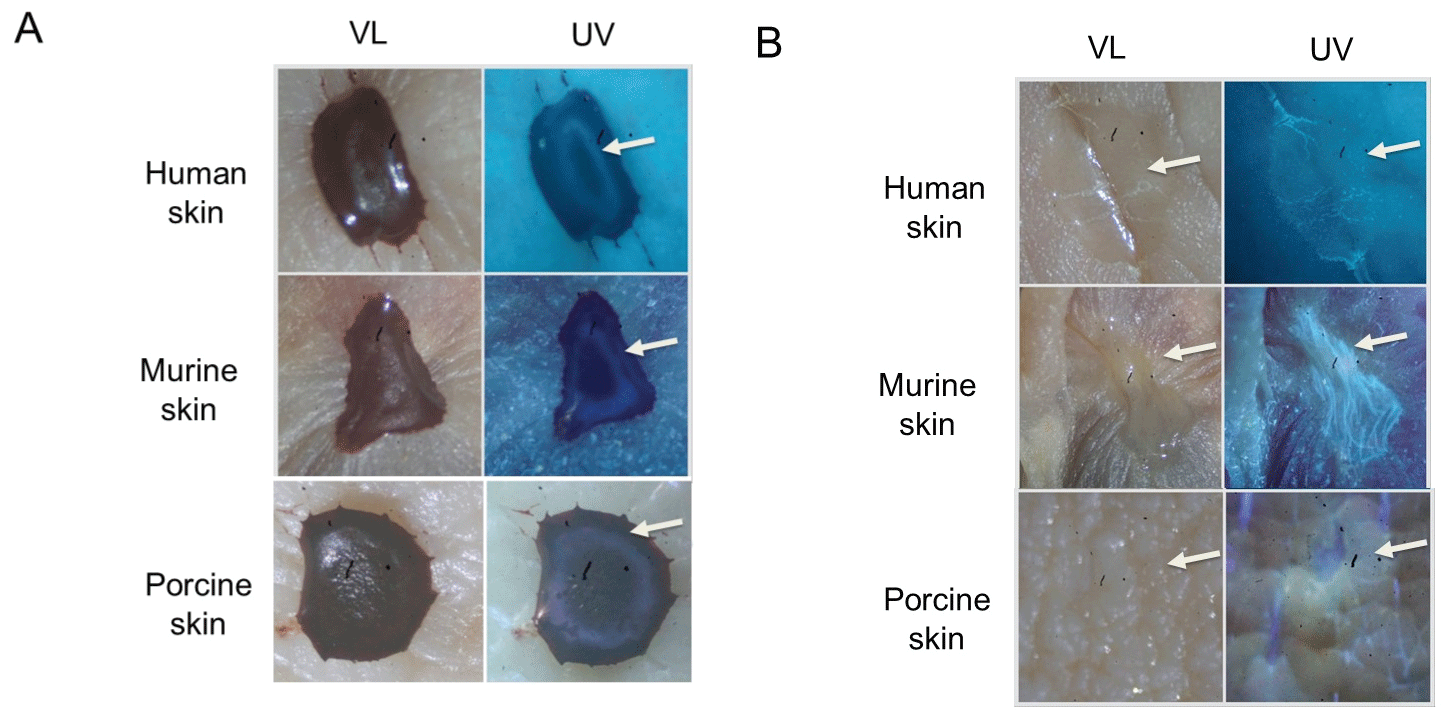
Analogous experiments to those done using glass were performed with drying or dried blood on the skin. As shown in Figure 4A, blood dried onto human, murine, and porcine skin had a similar appearance, with a distinct fluorescent serum halo present on all three skin types (Figure 4A, arrow). Isolated blood serum was visualized on all three skin types as well (Figure 4B, arrow). Like the observations using glass as the drying substrate, fresh blood on murine skin existed in the plasma blister stage in high humidity groups with all three temperatures (Figures 5A-C, upper right, UV). As with glass, only fresh blood on skin that was incubated under high humidity conditions was effectively transferred to filter paper after five hours (Figure 5D). Dried blood on the skin also gave similar results as glass, with no fluorescent serum halo present in high humidity groups at high temperatures (Figures 5C, bottom right, UV). The transfer ability of dried blood in low and high-humidity groups was also similar, except for dried blood incubated at low temperatures under high humidity. No blood imprinting was observed at five hours but did occur at seven hours (Figure 5D); similar results were observed using blood added to porcine skin (data not shown). When all samples were allowed to dry at room temperature under low humidity and rephotographed, fresh blood originally under high humidity conditions showed a more “frosted” appearance under ultraviolet as previously noted, with serum spread throughout the center (Figures 6A-C, upper right, UV). Relatedly, as before, a discernible fluorescent halo was absent in previously dried groups with high temperature and high humidity (Figures 6C, bottom right, UV).
Figure 4: Dried blood and blood serum on human, murine, and porcine skin. (A) Blood was added to the indicated skin type, allowed to dry, and photographed under visible light (VL) and ultraviolet light (UV). The arrow indicates the position of the fluorescent serum halo observed for all three skin types. (B) Same as in A, except that purified serum was used; the position of the serum is indicated by an arrow.
Figure 5: Plasma/serum pattern formation and transfer ability of blood on mouse skin under various temperature and humidity conditions. (A-C): Blood was placed on mouse skin, incubated for five hours, and immediately photographed under visible light (VL) and ultraviolet light (UV). See text for specific details. (D) Blood was cultured as above and placed in contact with a piece of filter paper to evaluate the transfer properties under the conditions used.
Figure 6: Serum pattern formation of blood on mouse skin under various temperature and humidity conditions. The samples in Figure 5A-C were allowed to dry at room temperature under low humidity and then rephotographed as above under visible light (VL) and ultraviolet light (UV). See text for specific details.
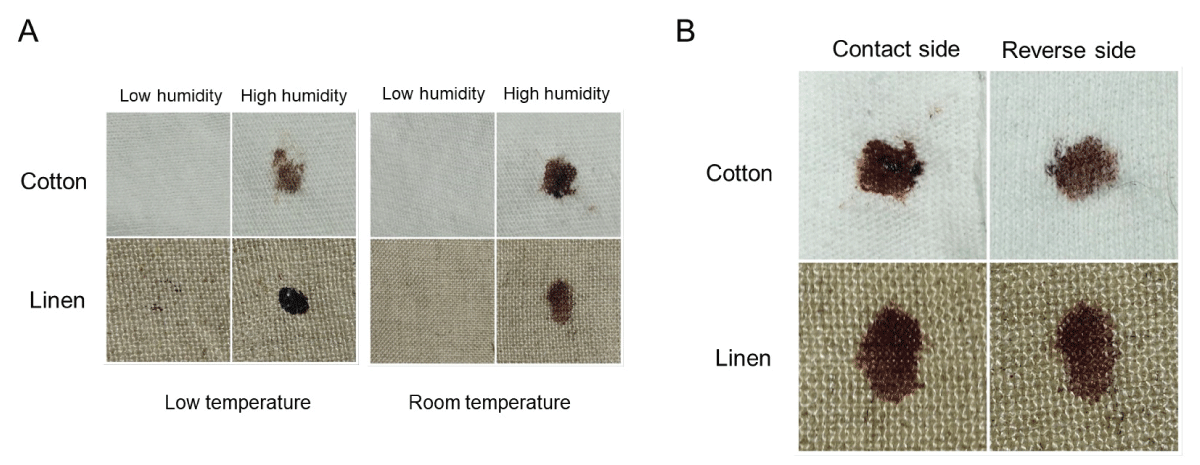
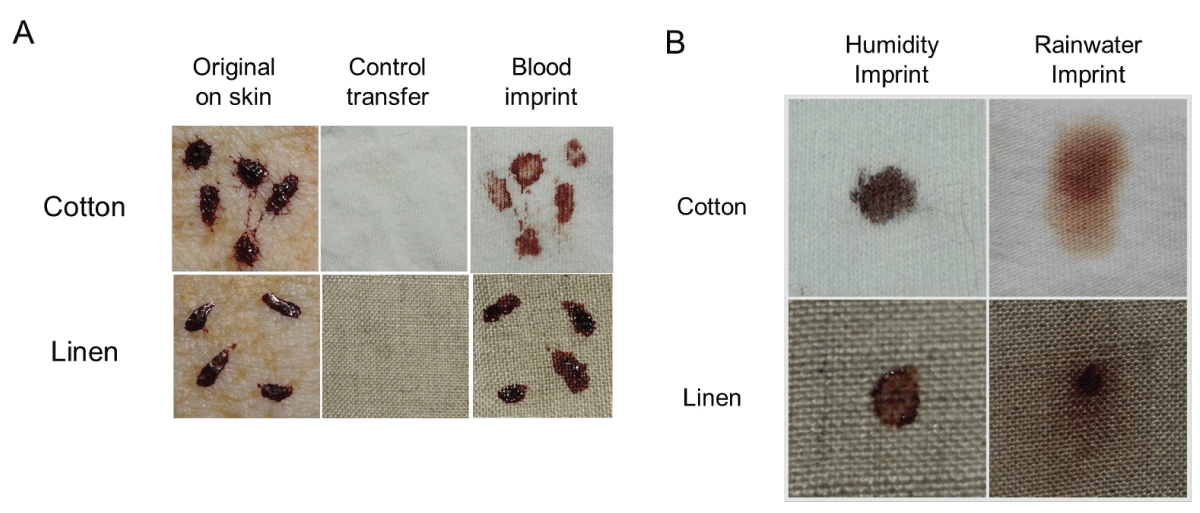
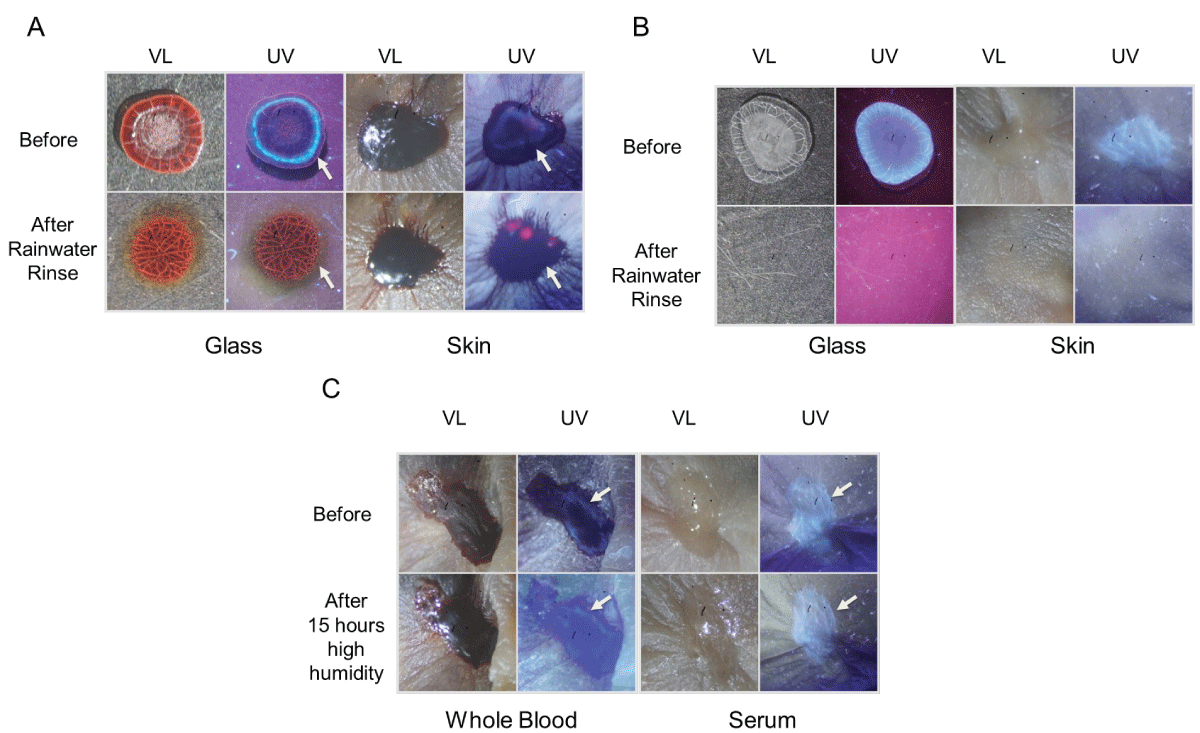
To expand on the observation that previously dried blood could become remoistened under high humidity conditions with restored transfer capability, studies were performed using two absorbent textiles (cotton and linen) that were overlaid onto skin to simulate clothing. A schematic of the experimental design is presented in Figure 7. As shown in Figure 8, blood imprinting was specific for high humidity conditions and occurred with both cotton and linen (Figure 8A); blood was absorbed sufficiently to soak through to the reverse side of both fabrics (Figure 8B). The general appearance of blood imprints was similar to the original blood pattern present on the skin (Figure 9A) and was noticeably different than what was observed using rainwater to gently moisten the outer portion of the fabric (Figure 9B). When a simulated rainwater rinsing was performed on blood-dried glass or skin, the fluorescent serum halo was clearly disturbed (Figure 10A). Similarly, exposure of isolated serum to rainwater abolished its detection (Figure 10B). In contrast to these findings, samples of whole blood or serum that had dried onto the skin and were incubated under high humidity with prolonged exposure (15 hours), fluorescent serum was still detectable (Figure 10C). These results suggest that, unlike exposure to rainwater, immersion in a high-humidity environment results in a more gradual exposure to moisture over time, allowing blood serum to persist. Additionally, these data demonstrate that high-humidity imprints provided a relatively accurate representation of the original bloodstain.
Figure 7: Schematic drawing showing the experimental setup for cloth overlay experiments. See the Material and Methods section for additional details.
Figure 8: Humidity imprinting of bloodstains onto absorbent textiles (A) Samples were overlaid with material as shown in Figure 7 and incubated for 12-15 hours under the indicated conditions. Material was removed, and the contact side was photographed. (B) Similar to (A), except that both contact and reverse sides are shown.
Figure 9: Humidity imprinting and the effect of rainwater exposure. (A) Samples were overlaid with material as presented in Figure 7 and incubated for 12-15 hours under high humidity at room temperature. The blood imprint is shown (right-hand side), together with the original bloodstain (left-hand side). A control transfer imprint is also included (middle) to demonstrate that the sample was completely dry prior to overlaying with the material. Note the congruence between the original bloodstain and the humidity imprint. (B) Dried blood samples were either imprinted under high humidity conditions at room temperature as in (A) or gently misted with rainwater on the overlaid cloth. The contact side of each imprint is shown.
Figure 10: The effect of rainwater exposure and high humidity on dried blood and blood serum. (A) Fresh blood was added to glass or skin, allowed to dry, and then gently misted with rainwater. Photographs were taken under visible light (VL) and ultraviolet light (UV) before and after rainwater exposure. The arrow indicates the position of the fluorescent serum halo, which is absent in rinsed samples. (B) Similar to (A), except that purified serum was used. (C) Blood or isolated serum was added to murine skin, allowed to dry, and then incubated under high humidity at room temperature for 15 hours. The position of the serum is indicated by an arrow.
Finally, it was of interest to determine if older dried blood was able to be sufficiently moistened for transfer. For these experiments, blood was added to parafilm and allowed to dry at room temperature (low humidity) for 3 days and 3 weeks. Samples were then exposed to low or high humidity conditions for five hours as before and touched to filter paper. As demonstrated, blood transfer was observed with high humidity, but not low humidity, groups for both 3-day and 3-week-old blood (Figure 11), supporting the idea that rewetting of dried blood is not limited to samples that are just a few hours old.
Figure 11: High humidity imprinting of 3-day and 3-weeks-old blood. Blood was added to parafilm and allowed to dry for the indicated period. Samples were then incubated under low or high humidity conditions for five hours and placed in contact with a piece of filter paper.
The current study provides evidence that serum pattern formation in drying and dried drops of blood are altered under conditions of high humidity. Additionally, these data show that dried blood may be rewetted sufficiently under these conditions such that blood transfer may occur.
Previous studies have established the distinct stages of blood drying on various surfaces, with monitoring of whole blood during the process [1-37]. Until recently, little was known regarding the migration and pattern formation of plasma in whole blood as its appearance is typically masked by red blood cells. Ultraviolet photography was used to provide the first documentation of plasma and serum pattern formation in drying blood drops on glass and skin, showing that distinct stages were present [40]. The data in the current study importantly extends these initial findings by showing that high humidity disrupts this process, with prolonged wetness and distinct, identifying features present in the dried bloodstain. These results suggest that ultraviolet examination of a victim’s bloodstains on a metal knife, a piece of glass, or their skin could indicate that the object/body had been stored under high humidity conditions while drying, e.g., inside of a refrigerator, a cave, a trunk with a damp interior, or a greenhouse. Identifying features for whole blood included a frosted appearance with no defined serum halo edge/ring at the periphery. Dried blood exposed to high humidity did not exhibit any abnormal morphological characteristics, except for bloodstains that were also exposed to high-temperature conditions; it was noted in this case that a distinct, fluorescent serum halo was absent at the periphery.
Although dried blood exposed to either low or high humidity was morphologically similar with the exception noted above, distinct differences existed in their transfer ability. The current study documents that blood exposed to high humidity became remoistened enough such that imprinting occurred on contact material. This observation occurred for all temperature ranges examined. Such findings could be relevant in cases where a victim was shot/stabbed at one location and hours/days later (after the blood had dried), moved to a different site with more humid conditions. Transfer of remoistened blood could explain discrepancies in the timeline and presence of (transferred) blood at the new location. Interestingly, it was found that 3-week-old blood dried onto parafilm could be sufficiently remoistened to acquire transfer ability, after just a short exposure to high humidity. It would be interesting to determine in future studies if blood and blood serum dried on a variety of materials exhibited the same property.
The effect of humidity exposure was markedly different than what was seen with other moisture exposure conditions, e.g., contact with rainwater. Even relatively small volumes of rainwater gently added (misted) to overlaying fabric resulted in more diffuse, smeared imprints than was observed with high humidity. It was also noted that serum stains dried on glass or skin were effectively removed with temperate rainwater exposure, which is not that surprising as blood plasma/serum is approximately 90 percent water in the liquid state. Thus, it is likely that dried, separated serum would be removed by even a gentle washing. A more gradual exposure to moisture, like a high-humidity environment, however, allows for serum stains to persist under these conditions.
Transfer of remoistened dried blood to the material under high humidity conditions provides an additional consideration for bloodstain analysis at a crime scene. As detailed above, blood or serum stains could imprint onto material significantly later than when the crime was initially committed, long after the blood had dried. For dried blood imprinting, the current studies focused on three different surface formats: murine skin, porcine skin, and parafilm. As extended incubation was not possible for the first two systems due to decomposition, the initial experiments utilized parafilm. Currently, no distinctions exist between fresh or dried blood that is imprinted; if specific features could be identified for each, this would allow further discrimination in classifying certain bloodstains that were rewetted and transferred after drying. Future efforts will include a more detailed, microscopic evaluation to determine if differences in warp and weft transfer patterns might exist. Additionally, it would be interesting to determine if high humidity imprinting is functional on blood that is years or even decades old; this might allow aged blood to be effectively solubilized from certain materials that have previously proven difficult to study.
In conclusion, this study shows that blood serum pattern formation was distinctly altered by increased humidity in drying drops of blood on glass or skin. In addition, these data document that high humidity conditions were sufficient to confer transfer ability on previously dried blood, maintaining the original bloodstain pattern.
Thank you to Barrie Schwortz and Hugh Farey for the helpful discussion. As always, thank you to my wife, Kathy, for everything.
- Hertaeg MJ, Tabor RF, Routh AF, Garnier G. Pattern formation in drying blood drops. Philos Trans A Math Phys Eng Sci. 2021 Aug 9;379(2203):20200391. doi: 10.1098/rsta.2020.0391. Epub 2021 Jun 21. PMID: 34148412; PMCID: PMC8405133.
- Chen R, Zhang L, Zang D, Shen W. Blood drop patterns: Formation and applications. Adv Colloid Interface Sci. 2016 May;231:1-14. doi: 10.1016/j.cis.2016.01.008. Epub 2016 Feb 4. PMID: 26988066.
- Brutin D, Sobac B, Loquet B, Sampol J. Pattern formation in drying drops of blood. J Fluid Mecha. 2011; 667: 85-95.
- San Pietro D, Steelberg R. A Preliminary Assessment of the Correlation of Drying Time and the Peripheral Rim Thickness of Perimeter Bloodstains. J Forensic Res. 2019; 10: 442-445.
- Denniff P, Woodford L, Spooner N. Effect of ambient humidity on the rate at which blood spots dry and the size of the spot produced. Bioanalysis. 2013 Aug;5(15):1863-71. doi: 10.4155/bio.13.137. PMID: 23905860.
- Deegan RD. Pattern formation in drying drops. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000 Jan;61(1):475-85. doi: 10.1103/physreve.61.475. PMID: 11046287.
- Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA. Capillary flow as the cause of ring stains from dried liquid drops. Nature. 1997; 389: 827–829.
- Martusevich AK, Zimin Y, Bochkareva A. Morphology of dried blood serum specimens of viral hepatitis. Hepatitis Mon. 2007; 7: 207–210.
- Pozrikidis C. Flipping of an adherent blood platelet over a substrate. J Fluid Mech. 2006; 568: 161-172.
- Shabalin VN, Shatokhina SN. Diagnostic markers in the structures of human biological liquids. Singapore Med J. 2007 May;48(5):440-6. PMID: 17453103.
- Peschel O, Kunz SN, Rothschild MA, Mützel E. Blood stain pattern analysis. Forensic Sci Med Pathol. 2011 Sep;7(3):257-70. doi: 10.1007/s12024-010-9198-1. Epub 2010 Nov 11. PMID: 21069481.
- Ramsthaler F, Schlote J, Wagner C, Fiscina J, Kettner M. The ring phenomenon of diluted blood droplets. Int J Legal Med. 2016 May;130(3):731-6. doi: 10.1007/s00414-015-1304-1. Epub 2015 Dec 30. PMID: 26718842.
- Bahmani L, Neysari M, Maleki M. The study of drying and pattern formation of whole human blood drops and the effect of thalassemia and neonatal jaundice on the patterns. Colloids Surf A. 2006; 513: 66–75.
- Yakhno TA, Yakhno VG, Sanin AG, Sanina OA, Pelyushenko AS, Egorova NA, Terentiev IG, Smetanina SV, Korochkina OV, Yashukova EV. The informative-capacity phenomenon of drying drops. IEEE Eng Med Biol Mag. 2005 Mar-Apr;24(2):96-104. doi: 10.1109/memb.2005.1411354. PMID: 15825851.
- Bou Zeid W, Brutin D. Influence of relative humidity on spreading, pattern formation and adhesion of a drying drop of whole blood. Colloids Surf A. 2013; 430: 1-7.
- Chhasatia VH, Joshi AS, Sun Y. Effect of relative humidity on contact angle and particle deposition morphology of an evaporating colloidal drop. Appl Phys Lett. 2010; 97: 231909.
- Bou-Zeid W, Brutin D. Effect of relative humidity on the spreading dynamics of sessile drops of blood. Colloids Surf A. 2014; 456: 273–285.
- Giorgiutti-Dauphiné F, Pauchard L. Drying drops: Drying drops containing solutes: From hydrodynamical to mechanical instabilities. Eur Phys J E Soft Matter. 2018 Mar 19;41(3):32. doi: 10.1140/epje/i2018-11639-2. PMID: 29546533.
- Brutin D, Starov V . Recent advances in droplet wetting and evaporation. Chem Soc Rev. 2018 Jan 22;47(2):558-585. doi: 10.1039/c6cs00902f. PMID: 29090296.
- Parsa M, Harmand S, Sefiane K. Mechanisms of pattern formation from dried sessile drops. Adv Colloid Interface Sci. 2018 Apr;254:22-47. doi: 10.1016/j.cis.2018.03.007. Epub 2018 Mar 24. PMID: 29628116.
- Kovalchuk NM, Trybala A, Starov VM. Evaporation of sessile droplets. Curr Opinion Colloid Interface Sci. 2014; 19: 336–342.
- Larson RG. Transport and deposition patterns in drying sessile droplets. AlChE J. 2014; 60: 1538–1571.
- Sobac B, Brutin D. Structural and evaporative evolutions in desiccating sessile drops of blood. Phys Rev E Stat. Nonlinear Soft Matter Phys. 2011; 84: 1-5.
- Kenner T. The measurement of blood density and its meaning. Basic Res Cardiol. 1989 Mar-Apr;84(2):111-24. doi: 10.1007/BF01907921. PMID: 2658951.
- Rosina J, Kvasnák E, Suta D, Kolárová H, Málek J, Krajci L. Temperature dependence of blood surface tension. Physiol Res. 2007;56 Suppl 1:S93-S98. doi: 10.33549/physiolres.931306. Epub 2007 May 31. PMID: 17552890.
- Hertaeg MJ, Tabor RF, Garnier G. Effect of protein adsorption on the radial wicking of blood droplets in paper. J Colloid Interface Sci. 2018 Oct 15;528:116-123. doi: 10.1016/j.jcis.2018.05.037. Epub 2018 May 23. PMID: 29843059.
- Goehring L, Clegg WJ, Routh AF. Solidification and ordering during directional drying of a colloidal dispersion. Langmuir. 2010 Jun 15;26(12):9269-75. doi: 10.1021/la100125v. PMID: 20229997.
- Farrant J, Woolgar AE. Human red cells under hypertonic conditions; a model system for investigating freezing damage. I. Sodium chloride. Cryobiology. 1972 Feb;9(1):9-15. doi: 10.1016/0011-2240(72)90003-x. PMID: 5059683.
- Vodolazskaya IV, Tarasevich YY. The model of drying sessile drop of colloidal solution. Mod Phys Lett B. 2011; 25: 1303–1310.
- Thiriet M. The Biology and Mechanics of Blood Flows. Berlin, Germany: Springer. 2008.
- Hertaeg MJ, Tabor RF, Berry JD, Garnier G. Radial Wicking of Biological Fluids in Paper. Langmuir. 2020 Jul 21;36(28):8209-8217. doi: 10.1021/acs.langmuir.0c01318. Epub 2020 Jul 7. PMID: 32574068.
- Routh AF, Russel WB. Horizontal drying fronts during solvent evaporation from latex films. AlChE J. 1998; 44: 2088–2098.
- Laan N, de Bruin KG, Slenter D, Wilhelm J, Jermy M, Bonn D. Bloodstain Pattern Analysis: implementation of a fluid dynamic model for position determination of victims. Sci Rep. 2015 Jun 22;5:11461. doi: 10.1038/srep11461. PMID: 26099070; PMCID: PMC4476491.
- Sant SP, Fairgrieve SI. Exsanguinated blood volume estimation using fractal analysis of digital images. J Forensic Sci. 2012 May;57(3):610-7. doi: 10.1111/j.1556-4029.2012.02056.x. Epub 2012 Feb 6. PMID: 22309183.
- Laan N, Bremmer RH, Aalders MC, de Bruin KG. Volume determination of fresh and dried bloodstains by means of optical coherence tomography. J Forensic Sci. 2014 Jan;59(1):34-41. doi: 10.1111/1556-4029.12272. Epub 2013 Oct 10. PMID: 24117600.
- De Gennes P, Brochard-Wyart F, Qu´er´e D, Capillarity and wetting phenomena: drops, bubbles, pearls, waves. Springer Science & Business Media. 2004.
- Riddel JP Jr, Aouizerat BE, Miaskowski C, Lillicrap DP. Theories of blood coagulation. J Pediatr Oncol Nurs. 2007 May-Jun;24(3):123-31. doi: 10.1177/1043454206298693. PMID: 17475978.
- Kearse KP. Ultraviolet 365 as an Alternative Light Source for Detection of Blood Serum. J Forensic Sci. 2020 Sep;65(5):1716-1721. doi: 10.1111/1556-4029.14439. Epub 2020 Apr 28. PMID: 32343369; PMCID: PMC7496641.
- Kearse KP. Environmental influence on blood serum detection using ultraviolet. J Forensic Sci Res. 2021; 5: 030-036.
- Kearse, Kelly P. Visualization of Plasma and Serum Pattern Formation in Drying Drops of Blood. ARC Journal of Forensic Science. 2022; 1: 1-5.