More Information
Submitted: April 11, 2023 | Approved: April 25, 2023 | Published: April 26, 2023
How to cite this article: Ahmed AB, Ayuba GI. Estimating minimum post-mortem interval in a Nigerian murder case using Chrysomya megacephala (Fabricius, 1794) (Diptera: Caliphoridae): The first use of forensic entomology. J Forensic Sci Res. 2023; 7: 011-016.
DOI: 10.29328/journal.jfsr.1001044
Copyright License: © 2023 Ahmed AB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Unsolved murder; Forensic entomology; Accumulated-degree-day; Minimum post-mortem interval; Nigeria
Abbreviations: ADH/ADD: Accumulated Degree Hours/Accumulated Degree Days; BDTH: Barau Dikko Teaching Hospital; CSmin: Crime Scene Minimum Temperature; CSmax: Crime Scene Maximum Temperature; KASU: Kaduna State University; KOH: Potassium Hydroxide; MTmin: Meteorological Station Minimum Temperature; MTmin: Meteorological Station Maximum Temperature; minPMI: Minimum Post-Mortem Interval; PMI: Post-Mortem Interval
Estimating minimum post-mortem interval in a Nigerian murder case using Chrysomya megacephala (Fabricius, 1794) (Diptera: Caliphoridae): The first use of forensic entomology
Ado-Baba Ahmed1,2,* and Godwin Iko Ayuba3
and Godwin Iko Ayuba3
1Department of Biology, University of the Gambia, School of Arts and Sciences, Brikama Campus, Gambia
2Department of Biological Sciences, Kaduna State University, P.M.B. 2339, Kaduna, Nigeria
3Anatomic Pathology and Forensic Medicine, Barau-Dikko Teaching Hospital, Kaduna State University, P.M.B. 2339, Kaduna, Nigeria
*Address for Correspondence: Ado-Baba Ahmed, Professor, Department of Biology, University of the Gambia, School of Arts and Sciences, Brikama Campus, Gambia, Email: [email protected]
Introduction: This paper presents the first application of forensic entomology in a murder investigation in Nigeria involving the remains of a 54-years victim, on January 9th, 2019 in a shaded wooded area in advanced decomposition, with no clear indication of the time of death.
Objectives: To estimate the minimum post-mortem interval of a 54-year-old corpse recovered in the advanced decomposition stage using the blowfly Chrysomya megacephala and the Advance-Degree-day (ADD) method.
Results: An autopsy report revealed multiple wounds to the forehead including a bullet hole. Dead embalmed dead maggots recovered from the body were identified as C. megacephala, and an accumulated degree-day model was used to estimate the minimum post-mortem interval. The findings revealed that the recovered larvae were still within the third-instar stage and had accumulated thermal energy between 58 hours (= 1.6 days, equivalent to 38.7 ADD) and 102 hours (= 2.8 days, equivalent to 68.0 ADD), suggesting that the body may have been exposed to insect activity between January 1st and 9th January 2019 after expanding the range to cater for some uncertainties.
Conclusion: In this Nigerian murder case, forensic entomology used the calliphorid species C. megacephala to estimate the minPMI to be between 2 and 9 days before the body was discovered, which translates to 1st - 9th January 2019 after consideration of some uncertainties and limitations. This confirmed the crucial role that insects play in providing valuable evidence to complement forensic pathological findings in homicides when conventional methods failed. Notwithstanding difficulties with employing insect evidence in forensic investigations in Nigeria, the application of this modern forensic technique has the potential to aid in the resolution of many unsolved murder cases and expedite the delivery of justice. The ability of law enforcement agencies in Nigeria to use the potential of insects in criminal investigations can be improved through collaborations and training with professionals from diverse professions.
The study of insects is applied to legal problems in forensic entomology. One of the most well-known of these uses is the examination of insects found on human corpses to help crime scene investigators determine the cause of death, the minimum post-mortem interval (minPMI), the presence of drugs, and whether the body was moved from the primary crime scene [1]. Gail Anderson [2] claims that after three days, insect evidence is often the most accurate and sometimes the only method of determining elapsed time after death.
Blow flies are in the family Calliphoridae, within the superfamily Oestroidea [3] that play crucial roles in disease transmission and public health: as myiasis agents, the female blow flies lay their eggs on an appropriate host, like a wound, where the eggs hatch into larvae that then burrow into the host’s tissue to feed, causing tissue damage that can result in secondary infections and serious health issues if left untreated [4]; as mechanical vectors, their larvae carry bacteria-laden pathogens on their bodies that they can potentially transmit to a host by penetrating its tissue [4-6]. The maggots (larvae) of many species in this family have also been used in medicine as wound healers in larval debridement therapy, removing infection and necrotic tissue while promoting the formation of granulation tissue [7]. Calliphorids have also been connected to the commercial recycling of organic garbage [8].
As usually the first visitors to a corpse after death [9,10], calliphorids are also used in forensic entomology [11], where they provide forensic evidence in cases involving death. This makes them crucial for post-mortem (minPMI) assessment.
Forensic entomology is unknown in most parts of Africa. It is only in [12] developed nations in Europe, America, Australasia, and Asia where it is valued and applied [12,13]. For instance, there is no report of insect evidence connected to actual criminal casework in Nigeria, and the only evidence of its existence is found in a few publications, mostly from experimental studies conducted on insects associated with decomposing animal models [13-15]. We use the Accumulated-Degree-Day approach to estimate the minimum post-mortem interval (minPIA) of a decaying corpse in Kaduna, northern Nigeria, based on the development of the blowfly, Chrysomya megacephala.
Description and handling of the body
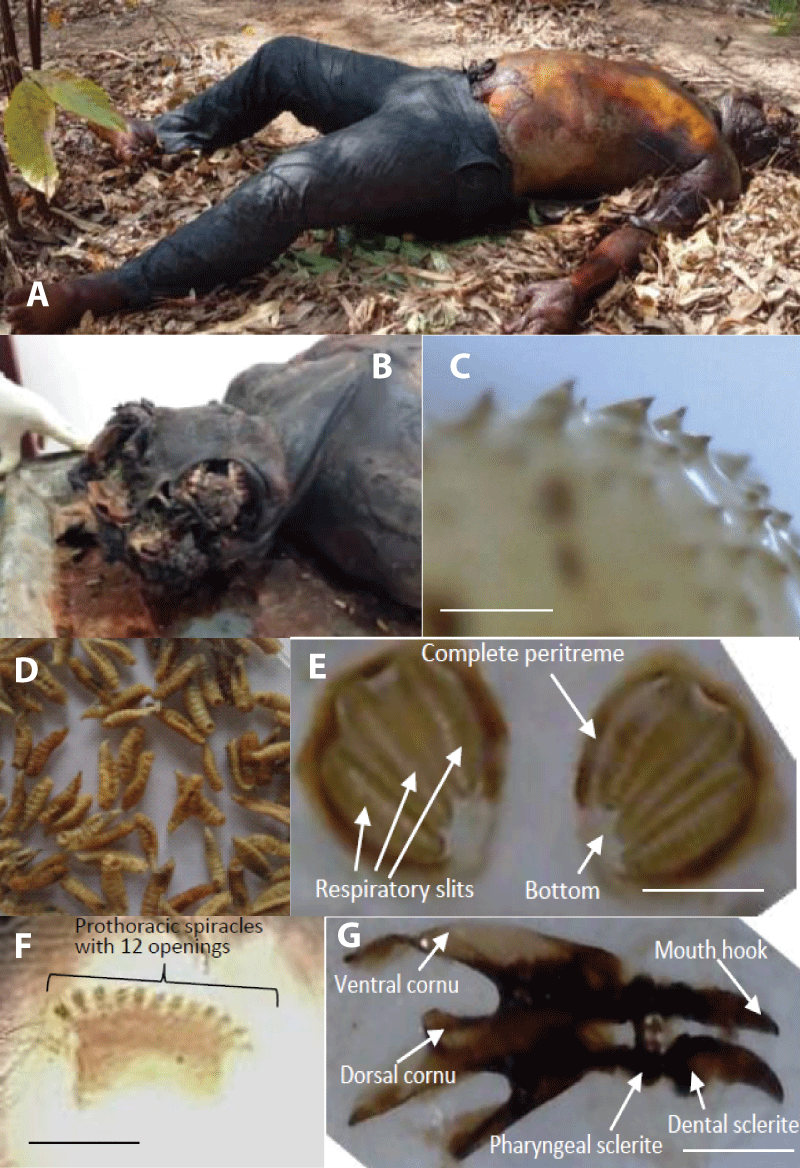
The body of a 54 years old male wearing only long trousers was found on 9th January 2019, lying face-up inside a deeply wooded area with trees creating intense shade (Figure 1A). The facial features were hardly recognizable as the head was fully decomposed, housing numerous maggots (Figure 1B).
The torso was bloated with desquamation of the skin, but little other evidence of decomposition externally (Figure 1A).
Figure 1: A: The body lying on its back. B: Close-up view of the decomposed head region. C: Bands of cuticular spines on the posterior dorsum between the prothorax and mesothorax. Each spine ended in one to three dark-pointed tips (only spines with single tips are shown here). D: Some of the recovered pre-migratory 3rd-instar maggots. E: A pair of the posterior spiracles of the pre-migratory third-instar maggots, with incomplete, heavily pigmented peritreme and relatively straight slits pointing to the button. The middle slit appears slightly bent inward. F: Prothoracic anterior spiracles with 12 openings of the pre-migratory third-instar maggots. G: Cephalopharyngeal skeletons of pre-migratory early 3rd-instar larva with the mouth hook and having longer dorsal cornua than the ventral cornua. Scale bars for E, F, G = 100 μm. Scale bar C = 1 cm, Magnification = 0.429x.
The body was removed to the mortuary of Barau Dikko Teaching Hospital, Kaduna State University by Police Officers on 10th January 2019 without collecting any insect evidence and embalmed immediately on arrival around 14:00 hr using 10% buffered saline (10% Formalin with 9% Normal saline). The autopsy report attributed death to a bullet wound in the forehead. Rigor, livor, and algor mortis were no longer applicable. The prime suspects had been taken into custody by the police and investigators were working to estimate the exact time of death to link it up with statements of the suspects and the time of the abduction. There was no physical evidence to link the suspects with the body. At this point, the medical pathologist requested entomological input to improve the overall accuracy of the estimation of the time of death.
Morphological identification of the insect species
When the body was discovered on 9th January 2019, there were no entomologists present to collect the insect evidence. Even though adult flies are easier to identify at the species level than immature stages, the opportunity to collect live maggots for rearing to adults was lost. Only embalmed dead maggots (Figure 1D) were recovered from the eye sockets of the body and preserved directly in 80% ethanol [16]. Each maggot was dissected at two sites using iridectomy scissors [17] under a stereo microscope [16,18]. The first cut is positioned across the middle of the second thoracic segment for viewing the internal cephalopharyngeal skeleton and external anterior spiracle. The second cut is positioned across the 11th body segment to observe the characteristics of the posterior spiracle. The dissected sections were soaked in acetic acid for 10 minutes to neutralize the KOH, and dehydrated by soaking them in an ascending series of ethanol (30%, 50%, 70% and 90%) for 30 minutes in each concentration. They were then soaked in absolute alcohol for 30 minutes, cleared in clove oil for 30 minutes, and soaked in xylene for 30 minutes. Once dehydrated, the specimens are transferred onto a glass slide containing xylene and mounting medium and covered with a cover slip, and observed under a light microscope. The cephalopharyngeal skeleton and posterior spiracle were observed and photographed using Dino-Lite Digital Microscope AM4115ZT (Figure 1C-G) [19].
Estimation of temperature at the time of the crime
A temperature Gemini Tinytag Plus2 Data logger was used to record the temperature at the scene for 5 days post-body discovery. The aim was to gather data that could be used to estimate the temperatures at the crime scene prior to the discovery of the body. The recorded temperature data (Maximum and Minimum) from the crime scene and the temperature data from the Kaduna Airport meteorological station were plotted in a linear regression graph and used to estimate what the temperature at the crime scene would have been at the time of the crime. This information is then used to estimate the age of the insect evidence found on the body (Table 1, Figure 1).
| Table 1: Determining the relationship between crime scene temperatures and meteorological station. | ||||||
| Date | Meteorological station ºC | Crime scene ºC | ||||
| MTmax | MTmin | MTAverage | CSmax | CSmin | CSAverage | |
| 10 Jan | 32 | 18 | 25 | 31.6 | 17.2 | 24.4 |
| 11 Jan | 30.1 | 20 | 25.05 | 29.1 | 18.7 | 23.9 |
| 12 Jan | 33.4 | 20.1 | 26.75 | 32.6 | 19 | 25.8 |
| 13 Jan | 32.8 | 19.9 | 26.35 | 31.1 | 19.8 | 25.45 |
| 14 Jan | 33.9 | 17.5 | 25.7 | 32.3 | 17.3 | 24.8 |
| MTx = Maximum Temperature Meteorological Station; MTmin: Minimum Temperature Meteorological Station; CSmax: Maximum Temperature Crime Scene; CSmin: Minimum Temperature Crime Scene | ||||||
Morphological identification of the maggots
The maggots were identified using literature and photo from Erzinclioglu [20] and Sukontason, et al. [21] to be members of the species Chrysomya megacephala (Diptera, Calliphoridae - blowflies), commonly known as the oriental latrine fly, based on morphological comparison of the dissected features.
Estimation of minimum post-mortem interval using ADD model
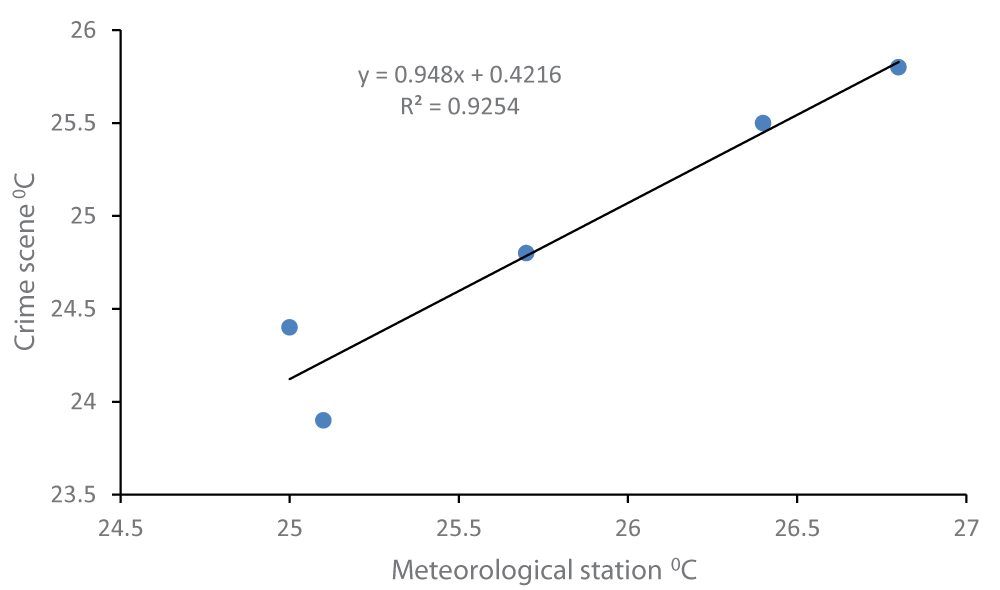
Since the temperatures of the crime scene were not known before the discovery of the body on 9th January 2019, and subsequent transfer to the mortuary on the next day, the maximum and minimum temperatures were obtained from the nearest weather station at Kaduna Airport, located at 15 km from the crime scene, and compared with that temperature at the crime scene [22]. A scatter plot gave the regression equation (crime scene temperature ºC = [0.948 x Kaduna Airport temperature ºC] + 0.422) and an R2 - value of 0.925 (Figure 2). The relatively high R2-value indicated a reasonably robust relationship between meteorological and crime scene temperature, therefore the equation was used as a correction factor [23] to estimate the ADH for each day when the body might have been present at the scene up to the discovery (Table 2).
Figure 2: Corrected crime scene temperature.
| Table 2: Computation of Accumulated Degree Days (ADD) at the crime scene for Chrysomya megacephala developing on the body. | ||||||
| Date | Meteorological | Estimated crime scene Temp ºC |
DD (Temp - Base Temp of 10.0 ºC) |
ADD | ||
| Max | Min | Avrg | ||||
| 10/Jan | 32.0 | 18.0 | 25.0 | 24.1 | 8.2 | 8.2 |
| 9/Jan | 31.0 | 17.0 | 24.0 | 23.2 | 13.2 | 24.4 |
| 8/Jan | 31.7 | 18.3 | 25.0 | 24.1 | 14.1 | 35.5 |
| 7/Jan | 31.1 | 17.9 | 24.5 | 23.6 | 13.6 | 49.1 |
| 6/Jan | 31.3 | 18.7 | 25.0 | 24.1 | 14.1 | 63.2 |
| 5/Jan | 31.8 | 18.2 | 25.0 | 24.1 | 14.1 | 77.3 |
| 4/Jan | 32.9 | 19.1 | 26.0 | 25.1 | 15.1 | 92.4 |
| 3/Jan | 32.0 | 17.0 | 24.5 | 23.6 | 13.6 | 106.0 |
| 2/Jan | 32.1 | 16.9 | 24.5 | 23.6 | 13.6 | 119.6 |
| 1/Jan | 32.4 | 16.6 | 24.5 | 23.6 | 13.6 | 133.2 |
| DD: Degree-Days; ADD: Accummulated Degree-Days; Avrg: Average | ||||||
Developmental studies of C. megacephala at 26 ºC indicate that this species requires 58hrs to reach the end of the 2nd instar and 102hrs to develop from to the end of the third-instar maggot, including the wandering stage [24]. However, the larvae were still 3rd-instar but not yet wandering as they were all found on the body. Therefore, they were likely to be somewhere between those two intervals (2-3 or 2-5 day age range). To calculate the ADD for C. megacephala, we use the development data from Prins [24], at a rearing temperature of 26 ºC, since it is almost the same as the mean of the corrected crime scene temperature (24.8 ºC) in Table 2.
From [24] Table 3, it takes 58 hours to reach the end of the 2nd instar and 102 hours to reach the end of the 3rd instar at 26 oC. However, the larvae recovered were still 3rd instars, still feeding on the body, so must be somewhere between those two intervals.
| Table 3: Development duration (hr) of egg and each larval instar of C. megacephala at 26 oC (Prins, 1982). |
|
| Development Stage | Development Duration (hrs) |
| Egg | 14.0 |
| 1st Instar Maggot | 23.0 |
| 2nd Instar Maggot | 21.0 |
| 3rd Instar Maggot | 44.0 |
| Pre-pupae | 60.0 |
ADD for end of 2nd larval stage/24 hr x (rearing temp in literature – base temp ºC).
ADD = 58/24 x (dev t ºC – base t ºC)
ADD = 2.42 x 16 = 38.7 ADD ºC
ADD for end of 3rd larval instar = Hours required to get to 3rd larval stage/24 hr x (rearing temp in literature – base temp ºC).
ADD = 102/24 x (dev t ºC – base t ºC)
ADD = 4.25 x 16 = 68.0 ADD ºC
Based on the evidence, it is likely that the C. megacephala maggots were in a transition developmental stage between the 2nd and 3rd instar, still actively feeding on the body at the time of embalming on January 10th. Taking into account this information, the estimated time of colonization can be determined to be between 58 hours (equivalent to 1.6 days or 38.7 accumulated degree days) and 102 degree days (equivalent to 2.8 days or 68.0 accumulated degree days).
These findings indicate that though the estimated age range of the larvae was most likely between 2 - 4 days, the body was likely subjected to insect activity between the dates of January 1st and 9th, 2019 after consideration of some uncertainties that may have played a role in the PMI estimation [25].
The current study identified the colonizing insect species as C. megacephala, which is widely dispersed throughout Africa and the New World [26]. Its presence was first noted in Ghana and Senegal in West Africa in 1977, but subsequently reported in Nigeria [14].
Estimating the post-mortem interval (minPMI) is crucial in investigating a suspicious death, as it provides detectives with a time frame to work with and helps in identifying potential suspects. For the first time in Nigeria, forensic entomology was applied in this case to determine the minimal PMI by examining the insects found on the victim’s body. This method proved to be helpful in the investigation of the murder.
A common technique used to determine the time of death is the accumulated degree hour or degree-day models (ADH/ADD), which was used in the current investigation to estimate the minPIA [27]. The method utilized the oldest life stage of the insect species found on the body to estimate the PMI, relying on its development rate. The developmental data used for estimating PMI for C. megacephala was obtained from South Africa [24], as there was no reference data available for Nigerian blow fly species. It is essential to acknowledge that the developmental rate of the South African population of the fly species may differ from the Nigerian population, as the literature does not cater to the local population.
Matuszewski [28] recently reviewed the challenges of estimating post-mortem intervals (PMI) using insect evidence. The review emphasized the importance of development reference data in determining the age of immature stages of insects found on a corpse. However, the review stressed the flaw in the use of data from the literature on populations of insects that occur in different geographic regions. He noted that even in central Europe, availability of developmental data is a challenge, with more information on cosmopolitan insect species that colonize corpses indoors, with certain species such as Lucilia sericata or Calliphora vicina receiving more attention than other species.
Another factor that affects the precision of PMI estimates is the quality of the insect evidence collected from the crime scene. In the present case, it is possible that older developmental stages such as puparia may have been missed by law enforcement officials who were not trained in forensic entomology and who quickly removed the body from the crime scene and failed to conduct a comprehensive search.
Blow flies are attracted to the orifices and open wounds on a corpse, and will typically lay their eggs in those areas first. In the current situation, the higher degree of decomposition in the head region caused by insect activity may not accurately indicate the manner of death, despite the fact that decomposition caused by insects is often used to determine the manner of death. The bullet wound in the head would not be considered a primary cause of death, but rather an additional injury. Even in the absence of injury, the head region, with its numerous orifices, can be a prime location for blowfly egg-laying, and as a result, the head may show advanced decomposition [29,30].
Legal professionals have connected Nigeria’s high rate of unsolved murder cases to the underuse of contemporary forensic methods, which has slowed down the prompt administration of justice [31,32]. The minPMI estimated in this case was derived sorely from insect evidence recovered from the body, otherwise, this case would have been added to the list of closed and inconclusive cases. These analyses have shown that Nigeria’s homicide investigation system needs to be updated to include contemporary scientific approaches including forensic entomology.
The need for collaboration in death investigations between experts from various fields has also been highlighted by this report. Entomologists were not contacted in the present instance until after the body and colonized maggots had been moved from the crime scene and embalmed, which is a sign that only a few people in crime scene investigation in Nigeria are familiar with the field of forensic entomology. The lack of familiarity among crime scene investigators in Nigeria, as highlighted in the report, is similar to the experience of Lutz, et al. [33] in Germany. Both instances indicated a lack of awareness of forensic entomology among professionals who are involved in death investigations, which often leads to insect evidence being overlooked or poorly handled, ultimately impacting negatively on the outcome of investigations. In addition to working together, investigators must be meticulous and professional in how they carry out their tasks. Hall [34] emphasized this, using the case of police officers who gathered insect larvae but failed to notice puparia that were apparent in photographs taken at the crime scene and compromised the investigation.
In this Nigerian murder case, forensic entomology used the calliphorid species C. megacephala to estimate the minimum post-mortem interval (minPMI) to be between 2 and 9 days before the body was discovered, which translates to 1st and 9th January 2019. This confirmed the crucial role that insects play in providing valuable evidence to complement forensic pathologists in homicides when conventional methods failed. Notwithstanding difficulties with employing insect evidence in forensic investigations in Nigeria, the application of this modern forensic technique has the potential to aid in the resolution of many unsolved murder cases and expedite the delivery of justice. The ability of law enforcement agencies in Nigeria to use the potential of insects in criminal investigations can be improved through collaborations and training with professionals from diverse professions.
The authors thank Dr. Martin Hall, NHM, United Kingdom for a thorough review of the manuscript. Technical support was provided by Mr. Solomon Bitrus from the Department of Biological Sciences at KASU, Nigeria. The mortuary staff of BDTH made significant contributions, for which the authors are appreciative.
- Tomberlin JK, Byrd JH. Forensic Entomology: International Dimensions and Frontiers. 2020.
- Anderson GS. Forensic entomology: The use of insects in death investigations. 2022. https://www.sfu.ca/~ganderso/forensicentomology.htm#_ftn1
- Yan L, Pape T, Meusemann K, Kutty SN, Meier R, Bayless KM, Zhang D. Monophyletic blowflies revealed by phylogenomics. BMC Biol. 2021 Oct 27;19(1):230. doi: 10.1186/s12915-021-01156-4. PMID: 34706743; PMCID: PMC8555136.
- Hall MJ, Wall RL, Stevens JR. Traumatic Myiasis: A Neglected Disease in a Changing World. Annu Rev Entomol. 2016;61:159-76. doi: 10.1146/annurev-ento-010715-023655. Epub 2015 Dec 7. PMID: 26667275.
- Wallman JF, LeBlanc HN. Medicolegal Forensic Entomology. In J Amendt, J Goff, M. Grassberger (Eds.), Current Concepts in Forensic Entomology. 2019; 295-318. Springer Cham.
- Pechal JL, Benbow ME, Tomberlin JK. Microbial ecology of carrion decomposition. In C. M Tridico & SM Williams (Eds.), Forensic Ecology Handbook: From Crime Scene to Court. 2020; 91-115. John Wiley & Sons.
- Sherman RA, Wetherbee MM, Belant JL. Consequences of Chronic Wasting Disease and Conservation Implications for North American Carnivores. Integrative Zoology. 2021; 16(4):819-828.
- Kallweit N, Rust MK. Insecticidal activity of spinosad and indoxacarb against larvae of Phormia regina (Diptera: Calliphoridae). Journal of Medical Entomology. 2021; 58(4): 1534-1540.
- Pohjoismäki JL, Karhunen PJ, Goebeler S, Saukko P, Sääksjärvi IE. Forensic entomology cases in Finland: A review of past and present cases. Forensic Sci Int. 2019; 297:259-267. doi: 10.1016/j.forsciint.2019.02.003. Epub 2019 Feb 7. PMID: 30802694.
- Singh D, Gurjar G, Sahni V, Garg R, Athawale V, Singh M, Singh D, Dohare R. Forensic entomology: An extensive review. Forensic Sci Int. 2020; 313:110334. doi: 10.1016/j.forsciint.2020.110334. Epub 2020 May 23. PMID: 32505854.
- Tarone AM, Crippen TL, Benbow ME. The Use of Calliphorid Flies in Forensic Investigations. In Carrion Ecology and Management. 2020; 149-165.
- Wells JD, LaMotte LR. Forensic Entomology. In StatPearls. 2021.
- Ewuim SC, Abajue MC. Forensic entomology: the journey so far in Nigeria. Open Sci. J. Biosci. Bioeng. 2016; 3(1):1-4.
- Onyishi GC, Osuala F, Aguzie IO, Okwuonu ES, Orakwelu CH. Arthropod succession on exposed and shaded mammalian carcasses in Nsukka, Nigeria. Animal Research International. 2020; 17(3):3869–3877.
- Akinkuolie TA, Kudirat AO. A review of forensic entomology in Nigeria: prospects and challenges. Journal of Forensic Sciences & Criminal Investigation. 2021; 18(3):555972.
- Sontigun N, Sukontason KL, Amendt J. Embalmed maggots (Diptera: Calliphoridae) in forensic entomology. Journal of Forensic Sciences. 2021; 66(1): 90-94.
- NHM. 2021. https://www.nhm.ac.uk/research-curation/scientific-resources/taxonomy-systematics/myiasis-larvae/specimen-collection-guide/index.html.
- Zaidi F, Zaidi S, Husain A. Forensic Entomology: Review of Various Aspects. Journal of Forensic Research and Analysis. 2021; 2(1):13-20.
- Wells JD, LaMotte LR, Sontigun N, Tarone AM. The value of calliphorid flies and their larvae in forensic entomology. Insects. 2021; 12(1):58. https://doi.org/10.3390/insects12010058
- Erzinclioglu YZ. The larvae of two closely-related blowfly species of the genus Chrysomya (Diptera, Calliphoridae). Entomologica Fennica. 3.XII. 1990.
- Sukontason K, Piangjai S, Siriwattanarungsee S, Sukontason KL. Morphology and developmental rate of blowflies Chrysomya megacephala and Chrysomya rufifacies in Thailand: application in forensic entomology. Parasitol Res. 2008 May;102(6):1207-16. doi: 10.1007/s00436-008-0895-6. Epub 2008 Feb 10. PMID: 18264799.
- Hofer IMJ, Hart AJ, Martín-Vega D, Hall MJR. Estimating crime scene temperatures from nearby meteorological station data. Forensic Sci Int. 2020 Jan;306:110028. doi: 10.1016/j.forsciint.2019.110028. Epub 2019 Oct 30. PMID: 31791700.
- Gennard D. Forensic Entomology: An Introduction. Wiley. Chichester. 2007; 224 ISBN: 9780470014783:240.
- Prins AJ. Morphological and Biological Note on Six South African Blowflies (Diptera, Calliphoridae) and Their Immature Stages. Ann. S. Afri. Mus. 1982; 90 (4):201-217.
- De Donno A, Di Vella G, Santoro V, Introna F. Factors affecting postmortem interval estimation. Journal of Forensic Sciences. 2019; 64(5):1368-1373. doi: 10.1111/1556-4029.14058.
- Badenhorst R, Villet MH. The uses of Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae) in forensic entomology. Forensic Sci Res. 2018 Mar 21;3(1):2-15. doi: 10.1080/20961790.2018.1426136. PMID: 30483647; PMCID: PMC6197084.
- Mostafa G, Shahanaz S. Estimation of accumulated degree hours-based post-mortem intervals in mammalian and avian model. Jahangirnagar University J. Biol. Sci. 2020; 9(1 & 2): 49-58.
- Matuszewski S. Post-Mortem Interval Estimation Based on Insect Evidence: Current Challenges. Insects. 2021 Apr 1;12(4):314. doi: 10.3390/insects12040314. PMID: 33915957; PMCID: PMC8066566.
- Campobasso CP, Introna F, Magni PA. Forensic entomology: application, utility and limitation in criminal investigations. Journal of forensic and legal medicine. 2014; 26:14-24.
- Smith KGV, Wells JD, Merritt RW, Tomberlin JK. Necrophagous insects on vertebrate remains in Texas: diversity, phenology, and decomposition ecology. Environmental Entomology. 2020; 49(2):368-379.
- Oluwatosin OM, Onwordi CT. The Challenges of Forensic Science in Nigeria. Journal of Forensic Sciences & Criminal Investigation. 2019; 11(1):555802. doi: 10.19080/jfsci.2019.11.555802
- Esimai OA, Esan OT. Overview of forensic science in Nigeria: trends, challenges and prospects. Journal of Forensic Science & Criminology. 2020; 8(1):555725. doi: 10.19080/JFSCJ.2020.08.555725
- Lutz L, Verhoff MA, Amendt J. To Be There or Not to Be There, That Is the Question-On the Problem of Delayed Sampling of Entomological Evidence. Insects. 2021 Feb 9;12(2):148. doi: 10.3390/insects12020148. PMID: 33572161; PMCID: PMC7915408.
- Hall MJR. The Relationship between Research and Casework in Forensic Entomology. Insects. 2021; 12:174. https://doi.org/10.3390/insects12020174