More Information
Submitted: March 06, 2023 | Approved: March 20, 2023 | Published: March 21, 2023
How to cite this article: Gesine Rempp A, Vill V. Requirement for object-oriented database management systems in forensic science. J Forensic Sci Res. 2023; 7: 001-003.
DOI: 10.29328/journal.jfsr.1001042
Copyright License: © 2023 Gesine Rempp A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Requirement for object-oriented database management systems in forensic science
Gesine Rempp A and Volkmar Vill*
Institute of Organic Chemistry, University of Hamburg, Germany
*Address for Correspondence: Volkmar Vill, Institute of Organic Chemistry, University of Hamburg, Germany, Email: [email protected]; [email protected]
The complex requirements of a database system for recording regulated chemicals exceed the capabilities of a relational database system. Inheritance, which is part of object-oriented programming, must also be logically transferred to chemical objects. This issue is illustrated here by means of examples of the Chemical Weapons Convention (CWC) and the German version of the New Psychoactive Substances Law (NpSG).
The use of certain chemicals for trading, industrial or personal purposes is regulated by international conventions and national laws, e.g. the UN conventions on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction (Chemical Weapons Convention, CWC) [1] or national narcotic drug laws, as well as environmental regulations [2]. If chemicals are part of a criminal investigation, it can be essential to identify which substances are regulated by which law.
Some of these regulations use single-substance lists. Many others use different identifiers [3] as well as varying levels of generalization as definitions of substance groups which are or can be displayed as Markush structures. Some of these substance definitions in regulatory texts include numbers of substances in the order of magnitude of tens of thousands of substances (for CWC 1A1) up to 1017 substances (for CWC 1A13, 1A14).
To keep the knowledge about chemicals adequately up-to-date and available, databases are reasonably used. An example of a requirement for a chemical database that can also apply chemical logic is the transfer of properties of substructures to the given overall structure, e. g. chromium (VI) and its salts [4a]. Some rules can also be calculated from the molecular formula, such as the gas volume in the case of complete combustion. Some properties were hard-coded, such as the structure definitions of the NpSG.
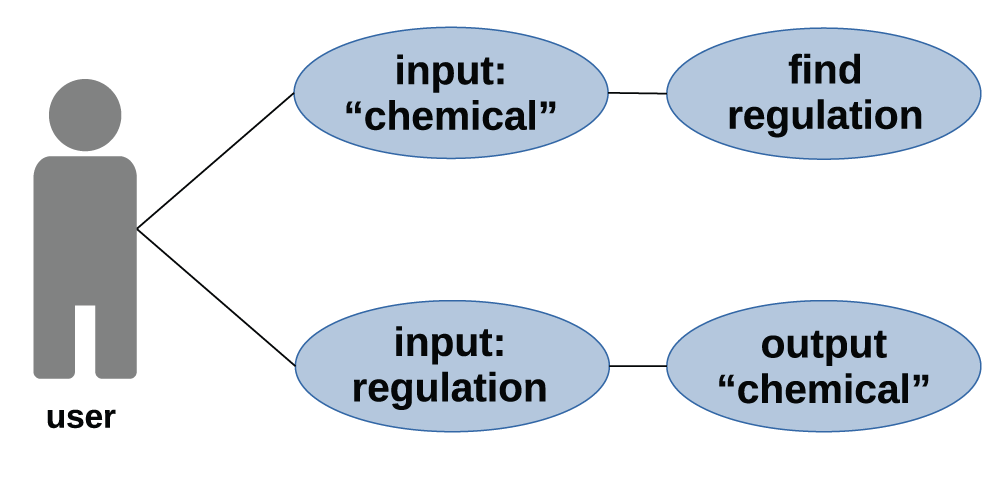
But the implementation of chemicals legislation can be challenging. But the more complex substance group definitions are usually not or not suitably represented in chemical databases. We define a use-case for this kind of database application as shown in Figure 1.Figure 1: use_case_law_en.pdf): use-case for finding chemicals to corresponding regulations and vice versa.
Substance definitions use different identifiers: chemical structure, biological effect, registry number, name, or regulation. These identifiers use different levels of abstraction or specifications.
For the implementation of complex substance group definitions based on the chemical structure, as some regulations use, the DBMS needs to be enabled to understand and interpret chemical structures and their generalized forms and match them with each other.
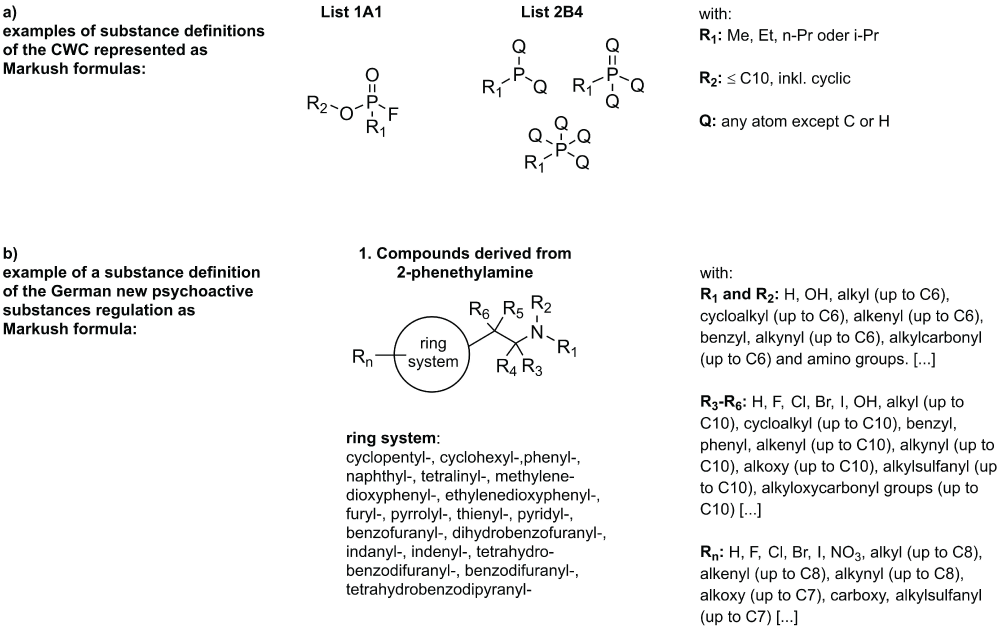
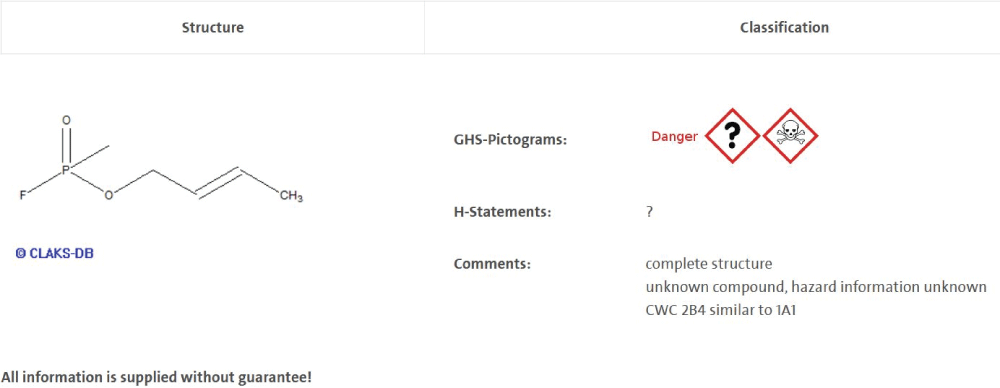
The CWC uses single substance definitions as well as group definitions [1,5] which can be displayed in a generalized form as Markus structures, as shown in Figure 2. A general applicable alkyl-substructure definition was established and used in the definition of substance groups of the CWC lists. It seemed reasonable for us to also provide information on the hazardous properties of these substances. Hence, the substances of list 1 of the CWC are generally considered toxic substances. List 2 of the CWC also contains some starting materials [1], rendering a general assignment as toxic material to all list 2 substances would be misleading. Since some of the structures in list 2 are very closely related to those in List A, we can assume that these structure analogs deserve classification as toxic (CWC 2B4 1A1 Analogue, Figure 3). The database applies this even if the substance is searched for is not registered and thus no other information is available.
Figure 2: subst_def_regulations.jpeg: Examples of different levels of complexity in substance definition in regulations. The substituent definitions in b) have been shortened for better readability.
Figure 3: Picture Screenshot OBW Crotylsarin.
The evolving problem of new psychoactive substances (NPS) [6,7] led to more complex chemical definitions in the field of narcotic drug regulation [8]. The German version of the new psychoactive substances regulation (NPSG) [2] is using a substance definition that was obviously inspired by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [7]. These more elaborate forms of Markush structures are basically a building block system for chemical structures as shown in Figure 2b). In the eventuality of a criminal case, however, this makes it complicated to understand whether or not there has been a violation of a law, especially for those who are not experts in the field of chemistry.
Nonetheless, the implementation of a database itself can help to verify the aims of the regulation as well as give an overview of the already regulated substances, as we were able to show in [9].
We found the approach with an object-relational database system is more suitable to represent the relations between substance groups. Substance groups can be interpreted as class definitions that can pass certain properties to their respective objects, in this case, substances. In this way, the principle of inheritance in object-oriented programming can become “chemical inheritance”. For example, substances that meet the definition of CWC 2B4, but are structural analogs of list 1A1, can inherit the attribute “toxic” from the latter, or substances that meet the criteria of the NPSG can be classified as a hazard to health [10].
An integrated information system for chemicals, chemical hazards, and forensic substances requires both human and artificial intelligence and cannot be created by simple SQL-based databases. A “chemical inheritance logic” needs to be included in class definitions of an object-orientated database system. Human intelligence is needed to create suitable substance definitions which can be used to summarize all individual compound descriptions. In the Online Classification of hazardous substances (OBW) [10,11] we show examples of the implementation of even complex substance definitions as the CWC and NPSG in a database based on object-relational programming concepts.
- Organization for the Prohibition of Chemical Weapons (OPCW): Conventions on the Prohibition of the Development, Production, Stockpiling, and Use of Chemical Weapons and their Destruction. 2023 March 03. https://www.opcw.org/chemical-weapons-convention/download-convention.
- Neue-psychoaktive-Stoffe-Gesetz (NpSG). 2023 Mar03. https://www.gesetze-im-internet.de/npsg/anlage.html
- Heller SR, McNaught A, Pletnev I, Stein S, Tchekhovskoi D. InChI, the IUPAC International Chemical Identifier. J Cheminform. 2015 May 30;7:23. doi: 10.1186/s13321-015-0068-4. PMID: 26136848; PMCID: PMC4486400.
- Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008 Jan;21(1):28-44. doi: 10.1021/tx700198a. Epub 2007 Oct 30. PMID: 17970581; PMCID: PMC2602826.
- Timperley CM, Forman JE, Abdollahi M, Al-Amri AS, Alonso IP, Baulig A, Borrett V, Cariño FA, Curty C, Gonzalez D, Kovarik Z, Martínez-Álvarez R, Mikulak R, Fusaro Mourão NM, Ramasami P, Neffe S, Raza SK, Rubaylo V, Takeuchi K, Tang C, Trifirò F, van Straten FM, Vanninen PS, Zaitsev V, Waqar F, Zina MS, Holen S, Weinstein HA. Advice from the Scientific Advisory Board of the Organisation for the Prohibition of Chemical Weapons on isotopically labeled chemicals and stereoisomers in relation to the Chemical Weapons Convention, Pure Appl. Chem., 2018; 90(10):1647-1670. https://doi.org/10.1515/pac-2018-0803
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA): Understanding the 'Spice' phenomenon, Thematic papers, 2009. Lisbon. 2023 March 03. https://www.emcdda.europa.eu/publications/thematic-papers/understanding-spice-phenomenon_en
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA): Synthetic cannabinoids in Europe, POD. 2023 Feb28; 2016:9. https://www.emcdda.europa.eu/topics/pods/synthetic-cannabinoids
- Eurojust and European Monitoring Centre for Drugs and Drug Addiction (EMCDDA): New psychoactive substances in Europe; Legislation and prosecution — current challenges and solutions. 2016; 30. https://doi.org/10.2810/777512
- Rempp GA, Voges LF, Vill V. Digitalisierung von Gesetzen: Ansatz für die Umsetzung des NpSG? - Definitionsunschärfen aus chemischer Sicht. Stoffrecht. 2023 March 03; 2020: 17 (1); 20-28. https://stoffr.lexxion.eu/article/STOFFR/2020/1/6
- Vill V. Online- Classification of hazardous substances (OBW). 2023 March 03. https://www.chemie.uni-hamburg.de/claks/obw/index_e.php
- Schmidt C, Vill V. An online system for the evaluation of hazardous substances, Chemistry Central Journal. 2009; 3: 1; 70. https://doi.org/10.1186/1752-153X-3-S1-P70