More Information
Submitted: January 31, 2022 | Approved: February 26, 2022 | Published: February 28, 2022
How to cite this article: Saha D, Dhabal S, Sen DJ. Forensic science deals with safety armour during warfare explosives. J Forensic Sci Res. 2022; 6: 024-041.
DOI: 10.29328/journal.jfsr.1001033
Copyright License: © 2022 Saha D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: TNT; RDX; NG; PETN; TATP; AN; EGDN; HMX; Tear gas; Phosgene; Mustard gas; Nerve gas; Lethal gas; Organophosphorus agents; Holocaust; Asphyxia
Forensic science deals with safety armour during warfare explosives
Dhananjoy Saha1, Sampa Dhabal2 and Dhrubo Jyoti Sen3*
1Deputy Director, Directorate of Technical Education, Bikash Bhavan, Salt Lake City, Kolkata‒700091, West Bengal, India
2Assistant Director, Forensic Science Laboratory, SVSPA, West Bengal, India
3Department of Pharmaceutical Chemistry, School of Pharmacy, Techno India University, Salt Lake City, Sector-V, EM-4/1, Kolkata-700091, West Bengal, India
*Address for Correspondence: Dr. Dhrubo Jyoti Sen, Department of Pharmaceutical Chemistry, School of Pharmacy, Techno India University, Salt Lake City, Sector-V, EM-4/1, Kolkata-700091, West Bengal, India, Email: [email protected]
Forensic analysis of explosives includes analysis of post-explosion residues, and detection and identification of traces of explosives on suspects’ hands, on clothing, and on other related items. Preliminary field tests may be used for screening the debris on the explosion site. They include commercially available explosive vapor detectors and chemical color tests. Like post-explosion residues, personal items suspected to contain traces of explosives and hand-swabs, are often heavily contaminated. It is therefore of major importance that the analytical procedures have to include good screening, clean-up, and extraction methods. The main explosives dealt with in this chapter include nitroaromatic explosives, such as 2,4,6-trinitrotoluene (TNT) and 2,4,6, N-tetranitro-N-methyl aniline (tetryl), nitrate esters, such as ethylene glycol dinitrate (EGDN), glycerol trinitrate (nitroglycerin, NG), and pentaerythritol tetranitrate (PETN), and nitramine explosives, such as 1,3,5- trinitro-1,3,5-triazacyclohexane, (RDX) and 1,3,5,7-tetranitro-1,3,5,7-tetrazacyclooctane (HMX), as well as mixtures containing one or more of these explosives. Additional explosives include triacetone triperoxide (TATP) and ammonium nitrate (AN), NH4NO3
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances. The potential energy stored in an explosive material may, for example, be chemical energy, such as nitroglycerin or grain dust, pressurized gas, such as a gas cylinder, aerosol can, or BLEVE, nuclear energy, such as in the fissile isotope’s uranium-235 and plutonium-239. Explosive materials may be categorized by the speed at which they expand [1].
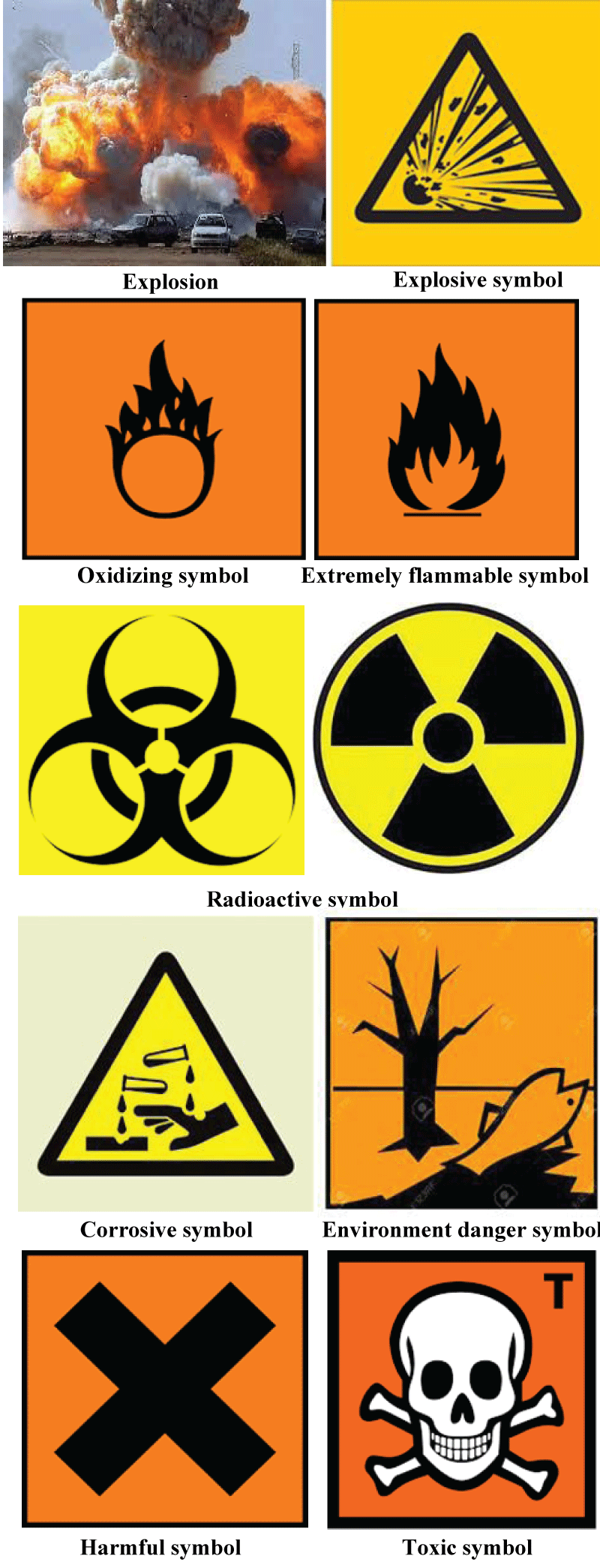
Harmful symbol toxic symbol
Figure 1: Symbols of toxicity.
Figure 1: Symbols of toxicity.
Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be “high explosives” and materials that deflagrate are said to be “low explosives”. Explosives may also be categorized by their sensitivity. Sensitive materials that can be initiated by a relatively small amount of heat or pressure are primary explosives and materials that are relatively insensitive are secondary or tertiary explosives. A wide variety of chemicals can explode; a smaller number are manufactured specifically for the purpose of being used as explosives. The remainder is too dangerous, sensitive, toxic, expensive, unstable, or prone to decomposition or degradation over short time spans. In contrast, some materials are merely combustible or flammable if they burn without exploding. The distinction, however, is not razor-sharp. Certain materials—dust, powders, gases, or volatile organic liquids—may be simply combustible or flammable under ordinary conditions, but become explosive in specific situations or forms, such as dispersed airborne clouds, confinement, or sudden release [2].
Chemical warfare (CW) involves using the toxic properties of chemical substances as weapons. This type of warfare is distinct from nuclear warfare and biological warfare, which together make up NBC, the military acronym for nuclear, biological, and chemical (warfare or weapons), all of which are considered “weapons of mass destruction” (WMDs). None of these falls under the term conventional weapons which are primarily effective due to their destructive potential. With proper protective equipment, training, and decontamination measures, the primary effects of chemical weapons can be overcome. Many nations possess vast stockpiles of weaponized agents in preparation for wartime use. The threat and the perceived threat have become strategic tools in planning both measures and counter-measures. A chemical weapon (CW) is a device that uses chemicals formulated to inflict death or harm on human beings. They are classified as weapons of mass destruction though they are separate from biological weapons (diseases), nuclear weapons, and radiological weapons (which use radioactive decay of elements). Chemical weapons can be widely dispersed in gas, liquid, and solid forms and may easily afflict others than the intended targets. Nerve gas, tear gas, and pepper spray are three modern examples. Lethal, unitary, chemical agents, and munitions are extremely volatile and they constitute a class of hazardous chemical weapons that are now being stockpiled by many nations. (Unitary agents are effective on their own and require no mixing with other agents.) The most dangerous of these are nerve agents GA, GB, and VX, and vesicant (blister) agents which are formulations of sulfur mustard such as H, HT, and HD. All are liquids at normal room temperature but become gaseous when released. Widely used during the First World War, the effects of so-called mustard gas, phosgene gas, and others caused lung searing, blindness, death, and maiming. Pepper spray is of common use today. It is potentially lethal. There are no recent records of pepper spray being used in the war despite the fact that it inflicts fewer injuries and has fewer side-effects if compared with impact and explosive weapons [3].
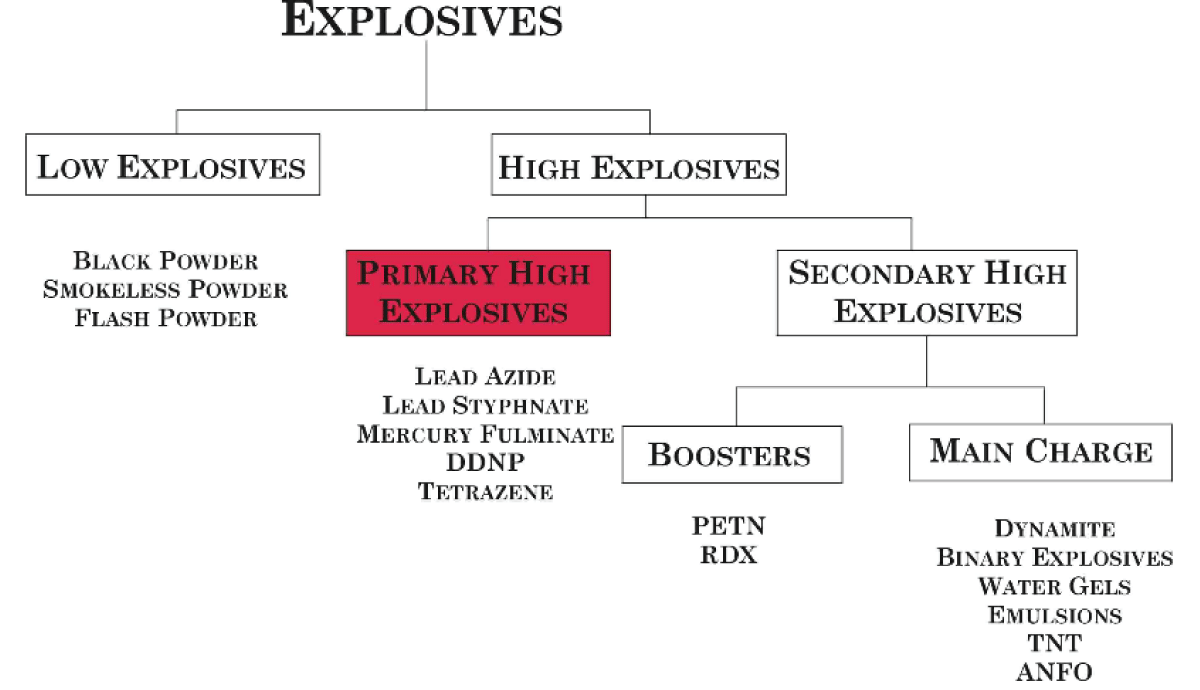
Figure 2: Classification of explosives.
Low explosion: Explosions that produce escaping gases of velocities less than the speed of sound.
High explosion: Explosions that produce escaping gases of velocities greater than the speed of sound [343 m/s].
Figure 2: Classification of explosives.
Classification
By sensitivity:
Primary: A primary explosive is an explosive that is extremely sensitive to stimuli such as impact, friction, heat, static electricity, or electromagnetic radiation. Some primary explosives are also known as contact explosives. A relatively small amount of energy is required for initiation. As a very general rule, primary explosives are considered to be those compounds that are more sensitive than PETN. As a practical measure, primary explosives are sufficiently sensitive that they can be reliably initiated with a blow from a hammer; however, PETN can also usually be initiated in this manner, so this is only a very broad guideline. Additionally, several compounds, such as nitrogen triiodide, are so sensitive that they cannot even be handled without detonating. Nitrogen triiodide is so sensitive that it can be reliably detonated by exposure to alpha radiation; it is the only explosive for which this is true. Primary explosives are often used in detonators or to trigger larger charges of less sensitive secondary explosives.
Figure 3: Primary Explosives.
Figure 3: Primary Explosives.
Primary explosives are commonly used in blasting caps and percussion caps to translate a physical shock signal. In other situations, different signals such as electrical or physical shock, or, in the case of laser detonation systems, light, are used to initiate an action, i.e., an explosion. A small quantity, usually milligrams, is sufficient to initiate a larger charge of explosive that is usually safer to handle [4].
Examples of primary high explosives are: Acetone peroxide, Alkali metal ozonide, Ammonium permanganate, Ammonium chlorate, Azidotetrazolates, Azoclathrates, Benzoyl peroxide, Benzvalene, 3,5-Bis(trinitromethyl)tetrazole, Chlorine oxides, Copper(I) acetylide, Copper(II) azide, Cumene hydroperoxide, CXP CycloProp(-2-)enyl Nitrate (or CPN), Cyanogen azide, Cyanuric triazide, Diacetyl peroxide, 1-Diazidocarbamoyl-5-azidotetrazole, Diazodinitrophenol Diazomethane, Diethylether peroxide, 4-Dimethylaminophenylpentazole, Disulfur dinitride, Ethyl azide, Explosive antimony, Fluorine perchlorate, Fulminic acid.
Halogen azides: Fluorine azide, Chlorine azide, Bromine azide, Iodine azide, Hexamethylene triperoxide diamine Hydrazoic acid, Hypofluorous acid, Lead azide, lead styphnate, Lead picrate, Manganese heptoxide, Mercury (II) fulminate, Mercury nitride, Methyl ethyl ketone peroxide, Nickel hydrazine nitrate, Nickel hydrazine perchlorate, Nitrogen trihalides: Nitrogen trichloride, Nitrogen tribromide, Nitrogen triiodide, Nitroglycerin, Nitronium perchlorate Nitrosyl perchlorate, Nitrotetrazolate-N-oxides Octaazacubane, Pentazenium hexafluoroarsenate, Peroxy acids, Peroxymonosulfuric acid, Selenium tetraazide, Silicon tetraazide, Silver azide, Silver acetylide, Silver fulminate, Silver nitride, Tellurium tetraazide, tert-Butyl hydroperoxide Tetraamine copper complexes, Tetraazidomethane, Tetrazene explosive, Tetranitratoxycarbon, Tetrazoles, Titanium tetraazide, Triazidomethane.
Oxides of xenon: Xenon dioxide, Xenon oxytetrafluoride, Xenon tetroxide, Xenon trioxide.
Secondary: A secondary explosive is less sensitive than a primary explosive and requires substantially more energy to be initiated. Because they are less sensitive, they are usable in a wider variety of applications and are safer to handle and store. Secondary explosives are used in larger quantities in an explosive train and are usually initiated by a smaller quantity of a primary explosive [5].
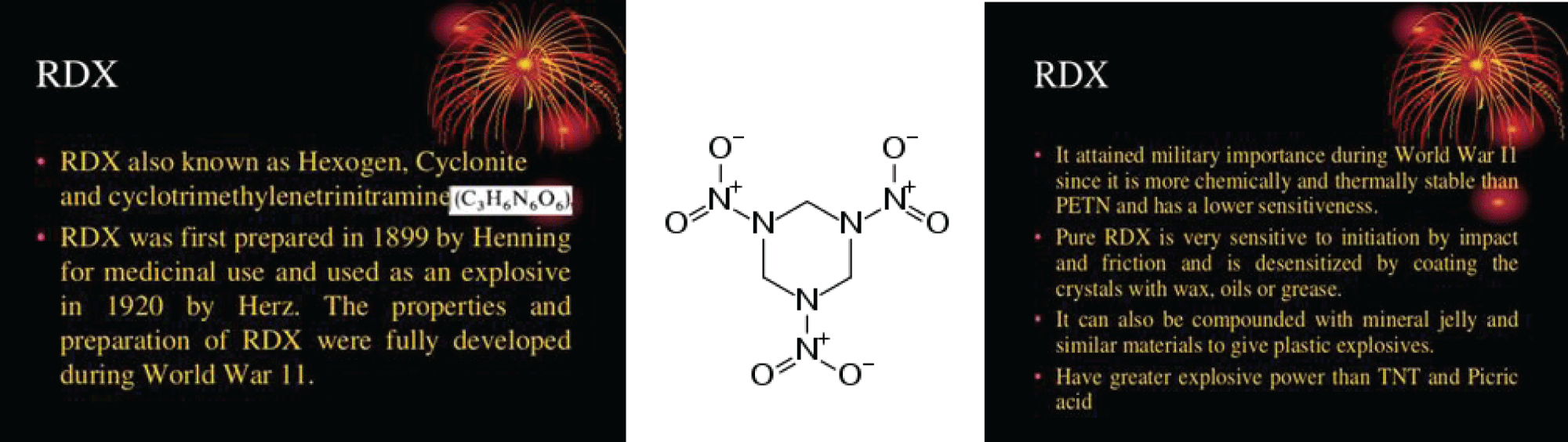
Examples of secondary explosives include TNT and RDX. RDX (abbreviation of “Research Department eXplosive” or “Royal Demolition eXplosive”), among other names (see the section Name), is an organic compound with the formula (O2N2CH2)3. It is a white solid without smell or taste, widely used as an explosive. Chemically, it is classified as a nitroamine alongside HMX, which is a more energetic explosive than TNT. It was used widely in World War II and remains common in military applications.
Figure 4: Secondary Explosives.
Figure 4: Secondary Explosives.
Figure 5: RDX.
Figure 5: RDX.
RDX [1,3,5-Trinitro-1,3,5-triazinane] is often used in mixtures with other explosives and plasticizers or phleg-matizers (desensitizers); it is the explosive agent in C-4 plastic explosives. It is stable in storage and is considered one of the most energetic blasts of the military high explosives, with a relative effectiveness factor of 1.60.
Tertiary: Tertiary explosives, also called blasting agents, are so insensitive to shock that they cannot be reliably detonated by practical quantities of primary explosive, and instead, require an intermediate explosive booster of secondary explosive. These are often used for safety and the typically lower costs of material and handling. The largest consumers are large-scale mining and construction operations.
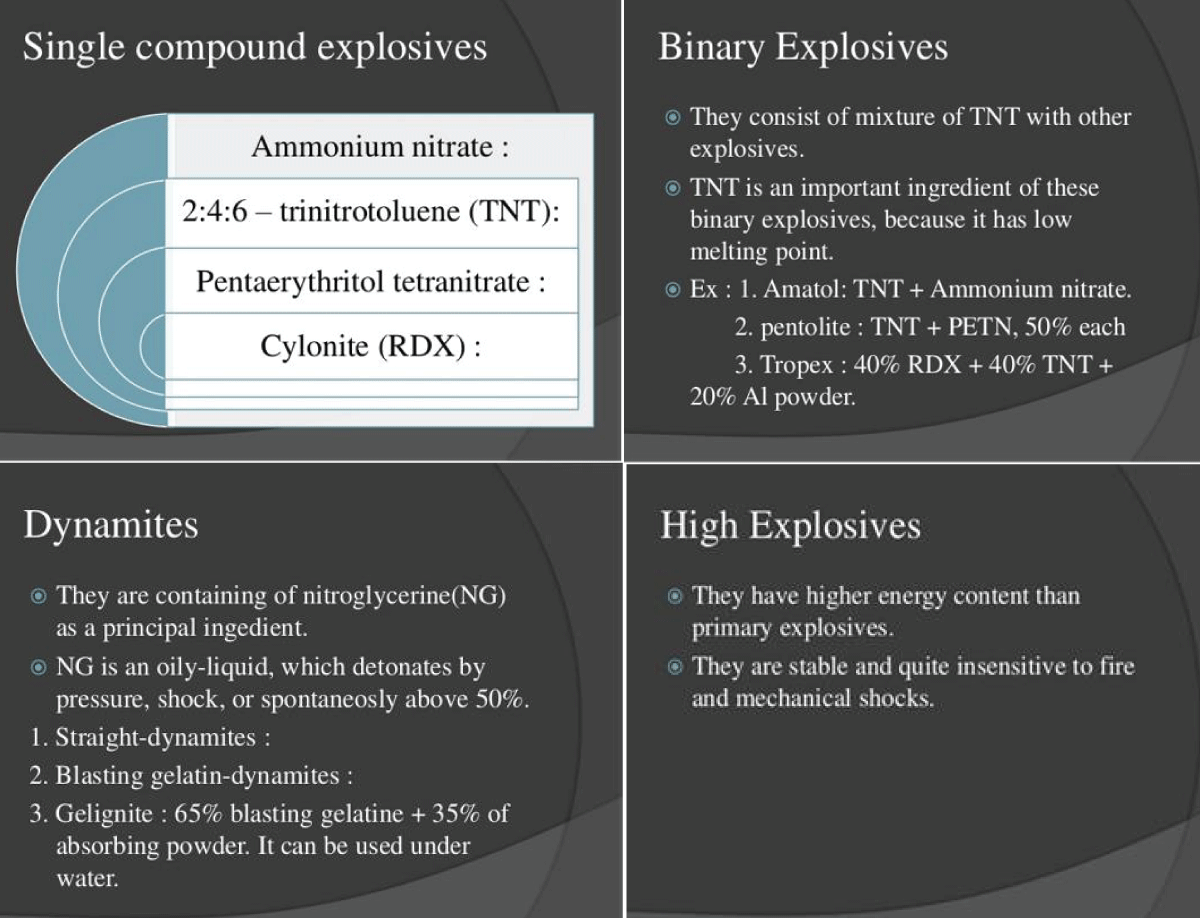
Most tertiaries include fuel and an oxidizer. ANFO can be a tertiary explosive if its reaction rate is slow. ANFO (/ˈænfoʊ/ AN-foh) (or AN/FO, for ammonium nitrate/fuel oil) is a widely used bulk industrial explosive. It consists of 94% porous prilled ammonium nitrate (NH4NO3) (AN), which acts as the oxidizing agent and absorbent for the fuel, and 6% number 2 fuel oil (FO). The use of ANFO originated in the 1950s. ANFO accounts for an estimated 90% of the 2.7 million tonnes (6 billion pounds) of explosives used annually in North America. It has found wide use in coal mining, quarrying, metal mining, and civil construction in applications where its low cost and ease of use may outweigh the benefits of other explosives, such as water resistance, oxygen balance, higher detonation velocity, or performance in small-diameter columns. ANFO is also widely used in avalanche hazard mitigation [6].
Figure 6: Tertiary Explosives.
Figure 6: Tertiary Explosivesns: not significant. (p > 0.05).
Cyanide gas: A gas chamber is an apparatus for killing humans or animals with gas, consisting of a sealed chamber into which a poisonous or asphyxiant gas is introduced. The most commonly used poisonous agent is hydrogen cyanide; carbon dioxide and carbon monoxide have also been used. Gas chambers were used as a method of execution for condemned prisoners in the United States beginning in the 1920s and continue to be a legal execution method in 3 states. During the Holocaust, large-scale gas chambers designed for mass killing were used by Nazi Germany as part of their genocide program and also by the Independent State of Croatia at the Jasenovac concentration camp. The use of gas chambers has also been reported in North Korea. When executions by gas chambers are conducted in the United States, the general protocol is as follows. First, the executioner will place a quantity of potassium cyanide (KCN) pellets into a compartment directly below the chair in the chamber. The condemned person is then brought into the chamber and strapped into the chair, and the airtight chamber is sealed. At this point, the executioner will pour a quantity of concentrated sulfuric acid (H2SO4) down a tube that leads to a smallholding tank directly below the compartment containing the cyanide pellets. The curtain is then opened, allowing the witnesses to observe the inside of the chamber [7].
The prison warden then asks the condemned individual if he or she wishes to make a final statement. Following this, the executioner(s) throws a switch/lever to cause the cyanide pellets to drop into the sulfuric acid, initiating a chemical reaction that generates hydrogen cyanide (HCN) gas: 2KCN(s) + H2SO4(aq) → 2HCN(g) + K2SO4(aq).
The gas is visible to the condemned, and he/she is advised to take several deep breaths to speed unconsciousness in order to prevent unnecessary suffering. Accordingly, execution by the gas chamber is especially unpleasant for the witnesses to the execution due to the physical responses exhibited by the condemned during the process of dying. These responses can be violent and can include convulsions and excessive drooling. Following the execution, the chamber is purged of the gas through special scrubbers and must be neutralized with anhydrous ammonia (NH3) before it can be opened.
Figure 7: Gas chamber.
Figure 7: Gas chamber.
Guards wearing oxygen masks remove the body from the chamber. Finally, the prison doctor examines the individual in order to officially declare that he or she is dead and release the body to the next of kin. One of the problems with the gas chamber is the inherent danger of dealing with such a toxic gas. Anhydrous ammonia is used to cleanse the chamber after cyanide gas has been used: HCN + NH3 → NH4+ + CN-.
The anhydrous ammonia used to clean the chamber afterward, and the contaminated acid that must be drained and disposed of, are both very poisonous. Nitrogen gas or oxygen-depleted air has been considered for human execution, as it can induce nitrogen asphyxiation. It has not been used to date [8].
Table 1: List of nerve gases.
| Table 1: List of nerve gases. | |||
| Name | First Use | Type | Usage |
| Xylyl bromide | 1914 | Lachrymatory, toxic | Both |
| Chlorine | 1915 | Corrosive. Lung Irritant | Both |
| Phosgene | 1915 | Irritant, Corrosive, toxic | Both |
| Benzyl bromide | 1915 | Lachrymatory | Central Powers |
| Chloromethyl chloroformate | 1915 | Irritant - Eyes, skin, lungs | Both |
| Trichloromethyl chloroformate | 1916 | Severe irritant causes burns | Both |
| Chloropicrin | 1916 | Irritant, lachrymatory, toxic | Both |
| Stannic chloride | 1916 | Severe irritant, causes asphyxiating | Allies |
| Bromoacetone | 1916 | Lachrymatory, irritant | Both |
| Acrolein | 1916 | Lachrymatory, toxic | Central Powers |
| Hydrogen cyanide (Prussic acid) | 1916 | Toxic, Chemical Asphyxiant | Allies |
| Hydrogen sulfide | 1916 | Irritant, toxic | Allies |
| Diphenylchloroarsine | 1917 | Irritant/Sternutatory (causes sneezing) | Central Powers |
| α-chlorotoluene (Benzyl chloride) | 1917 | Irritant, lachrymatory | Central Powers |
| Mustard gas | 1917 | Vesicant (blistering agent), a lung irritant | Both |
| Bis(chloromethyl) ether | 1918 | Irritant, can blur vision | Central Powers |
| Ethyldichloroarsine | 1918 | Vesicant | Central Powers |
| N-Ethylcarbazole | 1918 | Irritant | Central Powers |
| Blue Cross | 1917 | Toxic | Central Powers |
| Green Cross | 1915 | Toxic | Central Powers |
| Yellow Cross | 1916 | Toxic | Central Powers |
| White Cross | 1914 | Toxic | Central Powers |
Chemical weapons in World War I were primarily used to demoralize, injure and kill entrenched defenders, against whom the indiscriminate and generally slow-moving or static nature of gas clouds would be most effective. The types of weapons employed ranged from disabling chemicals, such as tear gas and severe mustard gas, to lethal agents like phosgene and chlorine. This chemical warfare was a major component of the first global war and the first total war of the 2025 century. The killing capacity of gas was limited, with four percent of combat deaths caused by gas. Gas was unlike most other weapons of the period because it was possible to develop effective countermeasures, such as gas masks. In the later stages of the war, as the use of gas increased, its overall effectiveness diminished. The widespread use of these agents of chemical warfare, and wartime advances in the composition of high explosives, gave rise to an occasionally expressed view of World War I as “the chemists’ war”.
1914: Tear gas: The earliest military uses of chemicals were tear-inducing irritants rather than fatal or disabling poisons. During the First World War, the French army was the first to employ gas, using 26 mm grenades filled with tear gas (ethyl bromoacetate, BrCH2COOC2H5) in August 1914. The small quantities of gas delivered, roughly 19 cm³ per cartridge, were not even detected by the Germans. The stocks were rapidly consumed and by November a new order was placed by the French military. As bromine was scarce among the Entente allies, the active ingredient was changed to chloroacetone (ClCH2COCH3). In October 1914, German troops fired fragmentation shells filled with a chemical irritant against British positions at Neuve Chapelle, though the concentration achieved was so small that it was barely noticed. None of the combatants considered the use of tear gas to be a conflict with the Hague Treaty of 1899, which prohibited the launching of projectiles containing asphyxiating or poisonous gas. Tear gas, also known as a lachrymator agent or lachrymator (from the Latin lacrima meaning “tear”), sometimes colloquially known as “mace” after an early commercial aerosol, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In addition, it can cause severe eye and respiratory pain, skin irritation, bleeding, and blindness. Common lachrymators both currently and formerly used as tear gas include pepper spray (OC gas), PAVA spray (nonivamide), CS gas, CR gas, CN gas (phenacyl chloride), bromoacetone, xylyl bromide, and Mace (a branded mixture) [9].
Figure 8: Tear gas.
Figure 8: Tear gas.
1915: Large-scale use and lethal gases: The first instance of large-scale use of gas as a weapon was on 31st January 1915, when Germany fired 18,000 artillery shells containing liquid xylyl bromide tear gas on Russian positions on the Rawka River, west of Warsaw during the Battle of Bolimov. However, instead of vaporizing, the chemical froze and failed to have the desired effect. The first killing agent employed by the German military was chlorine. Chlorine is a powerful irritant that can inflict damage to the eyes, nose, throat, and lungs. High concentrations and prolonged exposure, can cause death by asphyxiation. German chemical companies BASF, Hoechst, and Bayer (which formed the IG Farben conglomerate in 1925) had been producing chlorine as a by-product of their dye manufacturing. In cooperation with Fritz Haber of the Kaiser Wilhelm Institute for Chemistry in Berlin, they began developing methods of discharging chlorine gas against enemy trenches.
1915: More deadly gases: The deficiencies of chlorine were overcome with the introduction of phosgene, which was prepared by a group of French chemists led by Victor Grignard and first used by France in 1915. Colorless and having an odor likened to “moldy hay,” phosgene was difficult to detect, making it a more effective weapon. Although phosgene was sometimes used on its own, it was more often used mixed with an equal volume of chlorine, with the chlorine helping to spread the denser phosgene. The Allies called this combination White Star after the marking painted on shells containing the mixture. Phosgene was a potent killing agent, deadlier than chlorine. It had a potential drawback in that some of the symptoms of exposure took 24 hours or more to manifest. This meant that the victims were initially still capable of putting up a fight; although this could also mean that apparently fit troops would be incapacitated by the effects of the gas on the following day. 3 Although phosgene was never as notorious in the public consciousness as mustard gas, it killed far more people, about 85% of the 100,000 deaths caused by chemical weapons during World War I [10].
1917: Mustard Gas: The most widely-reported and, perhaps, the most effective gas of the First World War was mustard gas. It was a vesicant that was introduced by Germany in July 1917 prior to the Third Battle of Ypres. The Germans marked their shells yellow for mustard gas and green for chlorine and phosgene; hence they called the new gas Yellow Cross. It was known to the British as HS (Hun Stuff), while the French called it Yperite (named after Ypres). Mustard gas is not a particularly effective killing agent (though in high enough doses it is fatal) but can be used to harass and disable the enemy and pollute the battlefield.
Figure 9: Lethal & toxic effect of war gas.
Figure 9: Lethal & toxic effect of war gas.
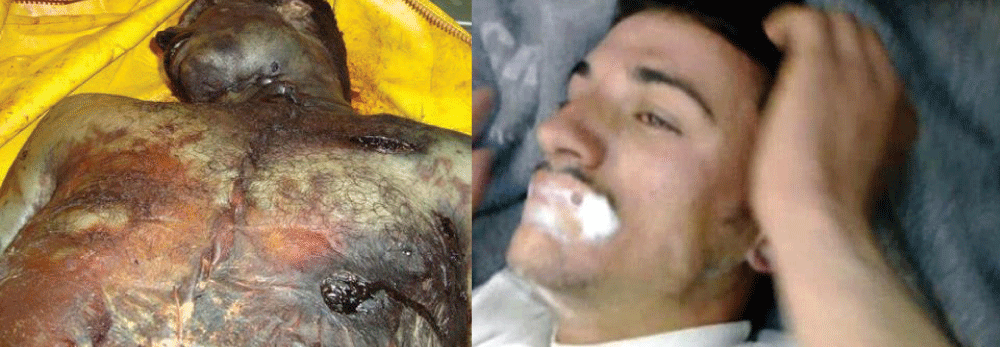
Delivered in artillery shells, mustard gas was heavier than air and it settled to the ground as an oily liquid resembling sherry. Once in the soil, mustard gas remained active for several days, weeks, or even months, depending on the weather conditions. The skin of victims of mustard gas blistered, their eyes became very sore and they began to vomit. Mustard gas caused internal and external bleeding and attacked the bronchial tubes, stripping off the mucous membrane. This was extremely painful. Fatally injured victims sometimes took four or five weeks to die of mustard gas exposure [11].
Nerve agents are a class of phosphorus-containing organic chemicals (organophosphates) that disrupt the mechanism by which nerves transfer messages to organs. The disruption is caused by blocking acetylcholinesterase, an enzyme that normally destroys acetylcholine, a neurotransmitter. As chemical weapons, they are classified as weapons of mass destruction by the United Nations according to UN Resolution 687 (passed in April 1991), and their production and stockpiling was outlawed by the Chemical Weapons Convention of 1993; the Chemical Weapons Convention officially took effect on April 29, 1997. The use of dangerous gases in warfare is forbidden by treaties already in the Hague Conventions of 1899 and 1907 and the Geneva Protocol of 1925. Poisoning by a nerve agent leads to contraction of pupils, profuse salivation, convulsions, involuntary urination and defecation, and eventual death by asphyxiation as control is lost over respiratory muscles. Some nerve agents are readily vaporized or aerosolized and the primary portal of entry into the body is the respiratory system. Nerve agents can also be absorbed through the skin, requiring that those likely to be subjected to such agents wear a full-body suit in addition to a respirator.
Sarin [Propan-2-yl methylphosphonofluoridate], or GB, is an organophosphorus compound with the formula (CH3)2CHO]CH3P(O)F. It is a colorless, odorless liquid, used as a chemical weapon owing to its extreme potency as a nerve agent. It has been classified as a weapon of mass destruction in UN Resolution 687. Production and stockpiling of sarin was outlawed by the Chemical Weapons Convention of 1993, and it is classified as a Schedule 1 substance. Sarin can be lethal even at very low concentrations, with death following within 1 to 10 minutes after direct inhalation due to suffocation from lung muscle paralysis, unless some antidotes, typically atropine or biperiden and pralidoxime, are quickly administered to a person. People who absorb a non-lethal dose, but do not receive immediate medical treatment, may suffer permanent neurological damage. Like other nerve agents, sarin attacks the nervous system by interfering with the re-absorption of neurotransmitters at neuromuscular junctions. Death will usually occur as a result of asphyxia due to the inability to control the muscles involved in breathing function. Specifically, sarin is a potent inhibitor of acetylcholinesterase, an enzyme that degrades the neurotransmitter acetylcholine after it is released into the synaptic cleft. In vertebrates, acetylcholine is the neurotransmitter used at the neuromuscular junction, where signals are transmitted between neurons from the central nervous systems to muscle fibers. Normally, acetylcholine is released from the neuron to stimulate the muscle, after which it is degraded by acetylcholinesterase, allowing the muscle to relax [12].
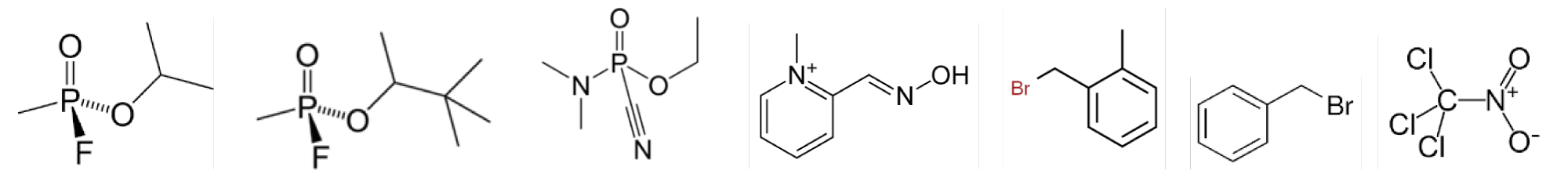
Figure 10: Sarin, Soman, Tabun, Pralidoxime, Xylyl bromide, Benzyl bromide, Chloropicrin.
Figure 10: Sarin, Soman, Tabun, Pralidoxime, Xylyl bromide, Benzyl bromide, Chloropicrin.
A build-up of acetylcholine in the synaptic cleft, due to the inhibition of cholinesterase, means the neurotransmitter continues to act on the muscle fiber so that any nerve impulses are effectively continually transmitted. Sarin acts on cholinesterase by forming a covalent bond with the particular serine residue at the active site. Fluoride is the leading group, and the resulting phosphoester is robust and biologically inactive. Its mechanism of action resembles that of some commonly used insecticides, such as malathion. In terms of biological activity, it resembles carbamate insecticides, such as Sevin, and the medicines pyridostigmine, neostigmine, and physostigmine [13].
Soman [3,3-Dimethylbutan-2-yl methylphosphonofluoridate], or GD (systematic name: O-Pinacolyl methylphosphonofluoridate), is an extremely toxic chemical substance. It is a nerve agent, that interferes with the normal functioning of the mammalian nervous system by inhibiting the cholinesterase enzyme. It is an inhibitor of both acetylcholinesterase and butyrylcholinesterase. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations according to UN Resolution 687. Its production is strictly controlled, and stockpiling is outlawed by the Chemical Weapons Convention of 1993 where it is classified as a Schedule 1 substance. Soman was the third of the so-called G-series nerve agents to be discovered along with GA (tabun), GB (sarin), and GF (cyclosarin). It is a volatile, corrosive, and colorless liquid with a faint odor when pure. More commonly, it is a yellow to brown color and has a strong odor described as similar to camphor. The LCt50 for soman is 70 mg·min/m3 in humans. It is both more lethal and more persistent than sarin or tabun, but less so than cyclosarin. GD can be thickened for use as a chemical spray using an acrylic copolymer.
Figure 11: Bombing of nerve gas shells.
Figure 11: Bombing of nerve gas shells.
It can also be deployed as a binary chemical weapon; its precursor chemicals are methylphosphonyl difluoride and a mixture of pinacolyl alcohol and amine. Soman is an organophosphorus nerve agent with a mechanism of action similar to Tabun. Nerve agents inhibit acetylcholine esterase (AChE) by forming an adduct with the enzyme via a serine residue on that enzyme. These adducts may be decomposed hydrolytically or, for example, by the action of some oximes and thereby regenerate the enzyme. A second reaction type, one in which the enzyme–organophosphate (OP) complex undergoes a subsequent reaction, is usually described as ‘‘aging’’. Once the enzyme–OP complex has aged it is no longer regenerated by the common, oxime reactivators. The rate of this process is dependent on the OP. Soman is an OP that stimulates the rate of aging most rapidly decreasing the half-life to just a few minutes. AChE is an enzyme involved with neurotransmission. Because of the severe decrease of the half-life of this enzyme, neurotransmission is abolished in a matter of minutes. Soman is a very effective compound that has severe health implications at very low doses. The LC50 of soman in the air is 70 mg min per m3. For compounds such as soman, which may also be used as a weapon, often a fraction of the LC50 dose is where the first effects appear. Miosis is one of the first symptoms of soman intoxication and can be seen in doses of less than 1% of the LC50. By using animal models, it is able to predict the LD50 value of soman. Most LD50 values via the same administration route give somewhat different lethal doses, which means the organisms metabolize the compounds differently [14].
Tabun or GA is an extremely toxic chemical substance. It is a clear, colorless, and tasteless liquid with a faint fruity odor. It is classified as a nerve agent because it fatally interferes with the normal functioning of the mammalian nervous system. Tabun [(RS)-Ethyl N, N-Dimethylphosphoramidocyanidate] or GA [Ethyl dimethylphosphoramidocyanidate] is an extremely toxic chemical substance. It is a clear, colorless, and tasteless liquid with a faint fruity odor. It is classified as a nerve agent because it fatally interferes with the normal functioning of the mammalian nervous system. Its production is strictly controlled and stockpiling outlawed by the Chemical Weapons Convention of 1993. Tabun is the first of the so-called G-series nerve agents along with GB (sarin), GD (soman), and GF (cyclosarin). Although pure tabun is clear, less-pure tabun may be brown. It is a volatile chemical, although less so than either sarin or soman. Tabun can be destroyed with bleaching powder, though the poisonous gas cyanogen chloride is produced.
The symptoms of exposure include nervousness/restlessness, miosis (contraction of the pupil), rhinorrhea (runny nose), excessive salivation, dyspnea (difficulty in breathing due to bronchoconstriction/secretions), sweating, bradycardia (slow heartbeat), loss of consciousness, convulsions, flaccid paralysis, loss of bladder and bowel control, apnea (breathing stopped) and lung blisters. The exact symptoms of overexposure are similar to those created by all nerve agents. Tabun is toxic even in minute doses. The number and severity of symptoms that appear vary according to the amount of the agent absorbed and the rate of entry of it into the body. Very small skin dosages sometimes cause local sweating and tremors accompanied by characteristically constricted pupils with few other effects. Tabun is about half as toxic as sarin by inhalation, but in very low concentrations it is more irritating to the eyes than sarin. Also, tabun breaks down slowly, which after repeated exposure can lead to build-up in the body. The effects of tabun appear slowly when tabun is absorbed through the skin rather than inhaled. A victim may absorb a lethal dose quickly, although death may be delayed for one to two hours. A person’s clothing can release the toxic chemical for up to 30 minutes after exposure. Inhaled lethal dosages kill in one to ten minutes, and liquid absorbed through the eyes kills almost as fast. However, people who experience mild to moderate exposure to tabun can recover completely, if treated almost as soon as exposure occurs. The LCt50 for tabun is about 400 mg-min/m3. Treatment for suspected tabun poisoning is often three injections of a nerve agent antidote, such as atropine. Pralidoxime chloride (2-PAM Cl) also works as an antidote; however, it must be administered within a period of from minutes to a few hours following exposure to be effective [15].
Biological effects: As their name suggests, nerve agents attack the nervous system of the human body. All such agents function the same way: by inhibiting the enzyme acetylcholinesterase, which is responsible for the breakdown of acetylcholine (ACh) in the synapse. ACh gives the signal for muscles to contract, preventing them from relaxing. Initial symptoms following exposure to nerve agents (like sarin) are a runny nose, tightness in the chest, and constriction of the pupils. Soon after, the victim will then have difficulty breathing and will experience nausea and drooling. As the victim continues to lose control of their bodily functions, they will involuntarily salivate, lacrimation, urinate, defecate, and experience gastrointestinal pain and vomiting. Blisters and burning of the eyes and/or lungs may also occur. This phase is followed by initially myoclonic jerks followed by status epilepticus. Death then comes via complete respiratory depression, most likely via the excessive peripheral activity at the neuromuscular junction of the diaphragm. The effects of nerve agents are very long-lasting and increase with successive exposures. Survivors of nerve agent poisoning almost invariably suffer chronic neurological damage. This neurological damage can also lead to continuing psychiatric effects.
Mechanism of action: Pralidoxime (2-pyridine aldoxime methyl chloride,) or 2-PAM, usually as the chloride or methiodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to combat poisoning by organophosphates or acetylcholinesterase inhibitors (nerve agents) in conjunction with atropine and diazepam. Pralidoxime is typically used in cases of organophosphate poisoning. The acetylcholinesterase enzyme has two parts to it. An acetylcholine molecule, bound at both ends to both sites of the enzyme, is cleaved in two to form acetic acid and choline. In organophosphate poisoning, an organophosphate bind to just one end of the acetylcholinesterase enzyme [the esoteric site], blocking its activity. Pralidoxime is able to attach to the other half [the unblocked, anionic site] of the acetylcholinesterase enzyme. It then binds to the organophosphate, the organophosphate changes conformation and loses its binding to the acetylcholinesterase enzyme. The conjoined poison/antidote then unbinds from the site, and thus regenerates the enzyme, which is now able to function again. After some time though, some inhibitors can develop a permanent bond with cholinesterase, known as aging, where oximes such as pralidoxime cannot reverse the bond. Pralidoxime is often used with atropine (a muscarinic antagonist) to help reduce the parasympathetic effects of organophosphate poisoning. Pralidoxime is only effective in organophosphate toxicity (i.e. it does not have an effect if the acetylcholinesterase enzyme is carbamylated, as occurs with neostigmine or physostigmine). Pralidoxime has an important role in reversing paralysis of the respiratory muscles but due to its poor blood-brain barrier penetration, it has little effect on centrally-mediated respiratory depression. This is why atropine, which has excellent blood-brain barrier penetration, is concomitantly administered with pralidoxime during the treatment of organophosphate poisoning. While the efficacy of atropine has been well-established, clinical experience with pralidoxime has led to widespread doubt about its efficacy in the treatment of Organophosphorus poisoning. When a normally functioning motor nerve is stimulated, it releases the neurotransmitter acetylcholine, which transmits the impulse to a muscle or organ. Once the impulse is sent, the enzyme acetylcholinesterase immediately breaks down the acetylcholine in order to allow the muscle or organ to relax. Nerve agents disrupt the nervous system by inhibiting the function of the enzyme acetylcholinesterase via forming a covalent bond where acetylcholine would break down (undergoes hydrolysis). Acetylcholine thus builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. This same action also occurs at the gland and organ levels, resulting in uncontrolled drooling, tearing of the eyes (lacrimation), and excess production of mucus from the nose (rhinorrhea). The structures of the complexes of Soman (one of the most toxic nerve agents) with acetylcholinesterase from Torpedo California have been solved by X-ray crystallography (PDB codes: 2wfz, 2wg0, 2wg1, and 1som). The mechanism of action of soman could be seen in the example of 2wfz [16].
Antidotes: Atropine and related anticholinergic drugs act as antidotes to nerve agent poisoning because they block acetylcholine receptors, but they are poisonous in their own right. (Some synthetic anticholinergics, such as biperiden may counteract the central symptoms of nerve agent poisoning better than atropine since they pass the blood-brain barrier better than atropine.) While these drugs will save the life of a person affected with nerve agents, that person may be incapacitated briefly or for an extended period, depending on the amount of exposure. The endpoint of atropine administration is the clearing of bronchial secretions. Atropine for field use by military personnel is often loaded in an autoinjector, for ease of use in stressful conditions. Pralidoxime chloride, also known as 2-PAM chloride, is also used as an antidote. Rather than counteracting the initial effects of the nerve agent on the nervous system like atropine, pralidoxime chloride reactivates the poisoned enzyme (acetylcholinesterase) by scavenging the phosphoryl group attached to the functional hydroxyl group of the enzyme. Though safer to use, it takes longer to act. Revival of acetylcholinesterase with pralidoxime chloride works more effectively on nicotinic receptors while blocking acetylcholine with atropine is more effective on muscarinic receptors. Often, severe cases of poisoning are treated with both drugs.
Xylyl bromide, also known as methyl benzyl bromide or T-stoff, is a poisonous organic chemical compound with the molecular formula C8H9Br, formerly used as tear gas. Physically it is a colorless liquid (melting point 21 °C) with a pleasant aromatic odor. Xylyl bromide is highly toxic, irritant and lachrymatory and has been incorporated in chemical weapons since the early months of World War I. Some commentators say the first use was in August 1914, when the French attacked German soldiers with tear gas grenades, but the agent used in that incident was more likely to be ethyl bromoacetate, which the French had tested before the war. The first extensive use of xylyl bromide was the firing by German forces of 18,000 “T-shells” at Russian positions in the Battle of Bolimów in January 1915. The shells were modified 15 cm (6 inches) artillery shells containing an explosive charge and c. 3 kg (7 lb) xylyl bromide. The attack was a complete failure because the winter weather was too cold to permit an effective aerosol, and the agent was either blown back towards the German lines, fell harmlessly to the ground, or was insufficiently concentrated to cause damage. A similar attack at Nieuwpoort in March 1915 was also unsuccessful [17].
Figure 12: Chlorine shell & Death Valley of nerve gas.
Figure 12: Chlorine shell & Death Valley of nerve gas.
Chlorine (Cl2) is a toxic gas that irritates the respiratory system. Because it is heavier than air, it tends to accumulate at the bottom of poorly ventilated spaces. Chlorine gas is a strong oxidizer, which may react with flammable materials. Chlorine is detectable with measuring devices in concentrations of as low as 0.2 parts per million (ppm), and by smell at 3 ppm. Coughing and vomiting may occur at 30 ppm and lung damage at 60 ppm. About 1000 ppm can be fatal after a few deep breaths of the gas. Breathing lower concentrations can aggravate the respiratory system, and exposure to the gas can irritate the eyes. The toxicity of chlorine comes from its oxidizing power. When chlorine is inhaled at concentrations above 30 ppm, it begins to react with water and cells, which change it into hydrochloric acid (HCl) and hypochlorous acid (HClO). When used at specified levels for water disinfection, the reaction of chlorine with water is not a major concern for human health. Other materials present in the water may generate disinfection by-products that are associated with negative effects on human health, however, the health risk is far lower than drinking undisinfected water.
Phosgene (COCl2) was synthesized by the British chemist John Davy (1790–1868) in 1812 by exposing a mixture of carbon monoxide and chlorine to sunlight. He named it “phosgene” in reference to the use of light to promote the reaction; from Greek, phos (light) and gene (born). It gradually became important in the chemical industry as the 19th century progressed, particularly in dye manufacturing [18].
Chemical warfare: Following the extensive use of phosgene gas in combat during World War I, it was stockpiled by various countries as part of their secret chemical weapons programs. In May 1928, eleven tons of phosgene escaped from a war surplus store in central Hamburg. 300 people were poisoned of whom 10 died. US Army phosgene identification poster from World War II. Phosgene was then only frequently used by the Imperial Japanese Army against the Chinese during the Second Sino-Japanese War. Gas weapons, such as phosgene, were produced by Unit 731 and authorized by specific orders given by Hirohito (Emperor Showa) himself, transmitted by the chief of staff of the army. For example, the Emperor authorized the use of toxic gas on 375 separate occasions during the battle of Wuhan from August to October 1938.
Safety: Phosgene is an insidious poison as the odor may not be noticed and symptoms may be slow to appear. The odor detection threshold for phosgene is 0.4 ppm, four times the threshold limit value. Its high toxicity arises from the action of the phosgene on the proteins in the pulmonary alveoli, the site of gas exchange: their damage disrupts the blood-air barrier, causing suffocation. It reacts with the amines of the proteins, causing cross-linking by the formation of urea-like linkages, in accord with the reactions discussed above. Phosgene detection badges are worn by those at risk of exposure. Sodium bicarbonate may be used to neutralize liquid spills of phosgene. Gaseous spills may be mitigated with ammonia.
Benzyl bromide, or α-bromotoluene, is an organic compound consisting of a benzene ring substituted with a bromomethyl group. It can be prepared by the bromination of toluene at room temperature in air, using manganese (IV) oxide as a heterogeneous catalyst. It is a colorless liquid that is decomposed slowly in water. Benzyl bromide is used in organic synthesis for the introduction of the benzyl protecting group for alcohols and carboxylic acids. Benzyl bromide is a strong lachrymator and is also intensely irritating to skin and mucous membranes. Because of these properties, it has been used as a war gas.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organ chlorine compound that is a widely used chemical building block. Benzyl chloride is an alkylating agent. Indicative of its high reactivity (relative to alkyl chlorides), benzyl chloride reacts with water in a hydrolysis reaction to form benzyl alcohol and hydrochloric acid. Since benzyl chloride is quite volatile at room temperature, it can easily reach the mucous membranes where hydrolysis takes place with the production of hydrochloric acid. This explains why benzyl chloride is a lachrymator and has been used as a war gas. It is also very irritating to the skin [19].
Chloromethyl chloroformate (ClCOOCH2Cl) is a chemical compound developed for use in chemical warfare in World War I. It is a tearing agent designed to cause temporary blindness. It is a colorless liquid with a penetrating, irritating odor. Diphosgene is a chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene but is more conveniently handled because it is a liquid, whereas phosgene is a gas. Diphosgene was originally developed as a pulmonary agent for chemical warfare, a few months after the first use of phosgene. It was used as a poison gas in artillery shells by Germany during World War I. The first recorded battlefield use was in May 1916. Diphosgene was developed because the vapors could destroy the filters in gas masks in use at the time.
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. Its chemical structural formula is Cl2CNO2. Chloropicrin can be absorbed systemically through inhalation, ingestion, and the skin. At high concentrations, it is severely irritating to the lungs, eyes, and skin. In World War I German forces used concentrated chloropicrin against Allied forces as tear gas. While not as lethal as other chemical weapons, it caused vomiting and forced Allied soldiers to remove their masks to vomit, exposing them to other, more toxic chemical gases used as weapons during the war.
Stannic chloride (SnCl4) was used as a chemical weapon in World War I, as it formed an irritating (but non-deadly) dense smoke on contact with air: it was substituted for by a mixture of silicon tetrachloride and titanium tetrachloride near the end of the war due to shortages of tin. It is also used in the glass container industry for making an external coating containing tin (IV) oxide which toughens the glass. It is a starting material for organotin compounds. Stannic chloride is used in chemical reactions with fuming (90%) nitric acid for the selective nitration of activated aromatic rings in the presence of inactivated ones.
Bromoacetone is an organic compound with the formula CH3COCH2Br. This colorless liquid is a lachrymatory agent.
Acrolein (systematic name: propenal, CH2=CH-CHO) is the simplest unsaturated aldehyde. It is a colorless liquid with a piercing, disagreeable, acrid smell. The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fat breaking down into acrolein. It is produced industrially from propylene and is mainly used as a biocide and a building block to other chemical compounds, such as the amino acid methionine. Acrolein is toxic and is a strong irritant for the skin, eyes, and nasal passages. The main metabolic pathway for acrolein is the alkylation of glutathione. The WHO suggests a “tolerable oral acrolein intake” of 7.5 μg/day per kilogram of body weight. Although acrolein occurs in French fries, the levels are only a few micrograms per kilogram. In response to occupational exposures to acrolein, the US Occupational Safety and Health Administration has set a permissible exposure limit at 0.1 ppm (0.25 mg/m3) at an eight-hour time-weighted average.
Hydrogen cyanide (HCN), sometimes called prussic acid, is an inorganic compound with the chemical formula HCN. It is a colorless, extremely poisonous liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valuable precursor to many chemical compounds ranging from polymers to pharmaceuticals. A hydrogen cyanide concentration of 300 mg/m3 in the air will kill a human within 10–60 minutes. A hydrogen cyanide concentration of 3500 ppm (about 3200 mg/m3) will kill a human in about one 1minute. The toxicity is caused by the cyanide ion, which halts cellular respiration by acting as a non-competitive inhibitor for an enzyme in mitochondria called cytochrome c oxidase. Hydrogen cyanide has been absorbed into a carrier for use as a pesticide. Under IG Farben’s brand name Zyklon B (German >Cyclone B, with the B standing for Blausäure - “Prussic Acid”), it was used in the German concentration camp mass killing during World War II (esp. in the Holocaust). The same product is currently made in the Czech Republic under the trademark “Uragan D2.” Hydrogen cyanide was also the agent used in gas chambers employed in judicial execution in some U.S. states, where it was produced during the execution by the action of sulfuric acid on an egg-sized mass of potassium cyanide. Hydrogen cyanide is commonly listed amongst chemical warfare agents known as blood agents. As a substance listed under Schedule 3 of the Chemical Weapons Convention as a potential weapon that has large-scale industrial uses, manufacturing plants in signatory countries that produce more than 30 tonnes per year must be declared to and can be inspected by, the Organisation for the Prohibition of Chemical Weapons. During the First World War, USA and Italy used hydrogen cyanide against the Central Powers in 1918. France had used it in combat already in 1916, but this proved to be ineffective due to physical conditions. Under the name prussic acid, HCN has been used as a killing agent in whaling harpoons. Hydrogen cyanide gas in air is explosive at concentrations over 5.6%. This is far above its toxicity level [20].
Hydrogen sulfide (H2S) is considered a broad-spectrum poison, meaning that it can poison several different systems in the body, although the nervous system is most affected. The toxicity of H2S is comparable with that of hydrogen cyanide or carbon monoxide. It forms a complex bond with iron in the mitochondrial cytochrome enzymes, thus preventing cellular respiration. Since hydrogen sulfide occurs naturally in the body, the environment, and the gut, enzymes exist in the body capable of detoxifying it by oxidation to (harmless) sulfate. Hence, low levels of hydrogen sulfide may be tolerated indefinitely. At some threshold level, believed to average around 300–350 ppm, the oxidative enzymes become overwhelmed. Many personal safety gas detectors, such as those used by utility, sewage, and petrochemical workers, are set to alarm at as low as 5 to 10 ppm and to go into high alarm at 15 ppm. A diagnostic clue of extreme poisoning by H2S is the discoloration of copper coins in the pockets of the victim. Treatment involves immediate inhalation of amyl nitrite, injections of sodium nitrite or administration of 4-dimethylaminoethanol in combination with inhalation of pure oxygen, administration of bronchodilators to overcome eventual bronchospasm, and in some cases hyperbaric oxygen therapy (HBOT). HBOT has clinical and anecdotal support. Exposure to lower concentrations can result in eye irritation, a sore throat and cough, nausea, shortness of breath, and fluid in the lungs (pulmonary edema). These effects are believed to be due to the fact that hydrogen sulfide combines with alkali present in moist surface tissues to form sodium sulfide, a caustic. These symptoms usually go away in a few weeks. Long-term, low-level exposure may result in fatigue, loss of appetite, headaches, irritability, poor memory, and dizziness. Chronic exposure to low-level H2S (around 2 ppm) has been implicated in increased miscarriage and reproductive health issues among Russian and Finnish wood pulp workers, but the reports have not (as of circa 1995) been replicated. Short-term, high-level exposure can induce immediate collapse, with a high probability of death. If death does not occur, studies have shown that, in humans, high exposure to hydrogen sulfide can lead to cortical pseudo laminar necrosis and degeneration of the basal ganglia. It has also been shown that Cerebral edema has been the cause of death in some fatal exposures with hydrogen sulfide [21].
- 0.00047 ppm or 0.47 ppb is the odor threshold, the point at which 50% of a human panel can detect the presence of the compound.
- 0.0047 ppm is the recognition threshold, the concentration at which 50% of humans can detect the characteristic odor of hydrogen sulfide, normally described as resembling "a rotten egg".
- OSHA has established a permissible exposure limit (PEL) (8 hour time-weighted average (TWA)) of 10 ppm.
- 10–20 ppm is the borderline concentration for eye irritation.
- 20 ppm is the acceptable ceiling concentration established by OSHA.
- 50 ppm is the acceptable maximum peak above the ceiling concentration for an 8 hour shift, with a maximum duration of 10 minutes.
- 50–100 ppm leads to eye damage.
- At 100–150 ppm the olfactory nerve is paralyzed after a few inhalations, and the sense of smell disappears, often together with an awareness of danger.
- 320–530 ppm leads to pulmonary edema with the possibility of death.
- 530–1000 ppm causes strong stimulation of the central nervous system and rapid breathing, leading to loss of breathing.
- 800 ppm is the lethal concentration for 50% of humans for 5 minutes exposure (LC50).
- Concentrations over 1000 ppm cause immediate collapse with loss of breathing, even after inhalation of a single breath. Cortical pseudo laminar necrosis, degeneration of the basal ganglia, and cerebral edema have also been shown.
Although respiratory paralysis may be immediate, it can also be delayed up to 72 hours [22].
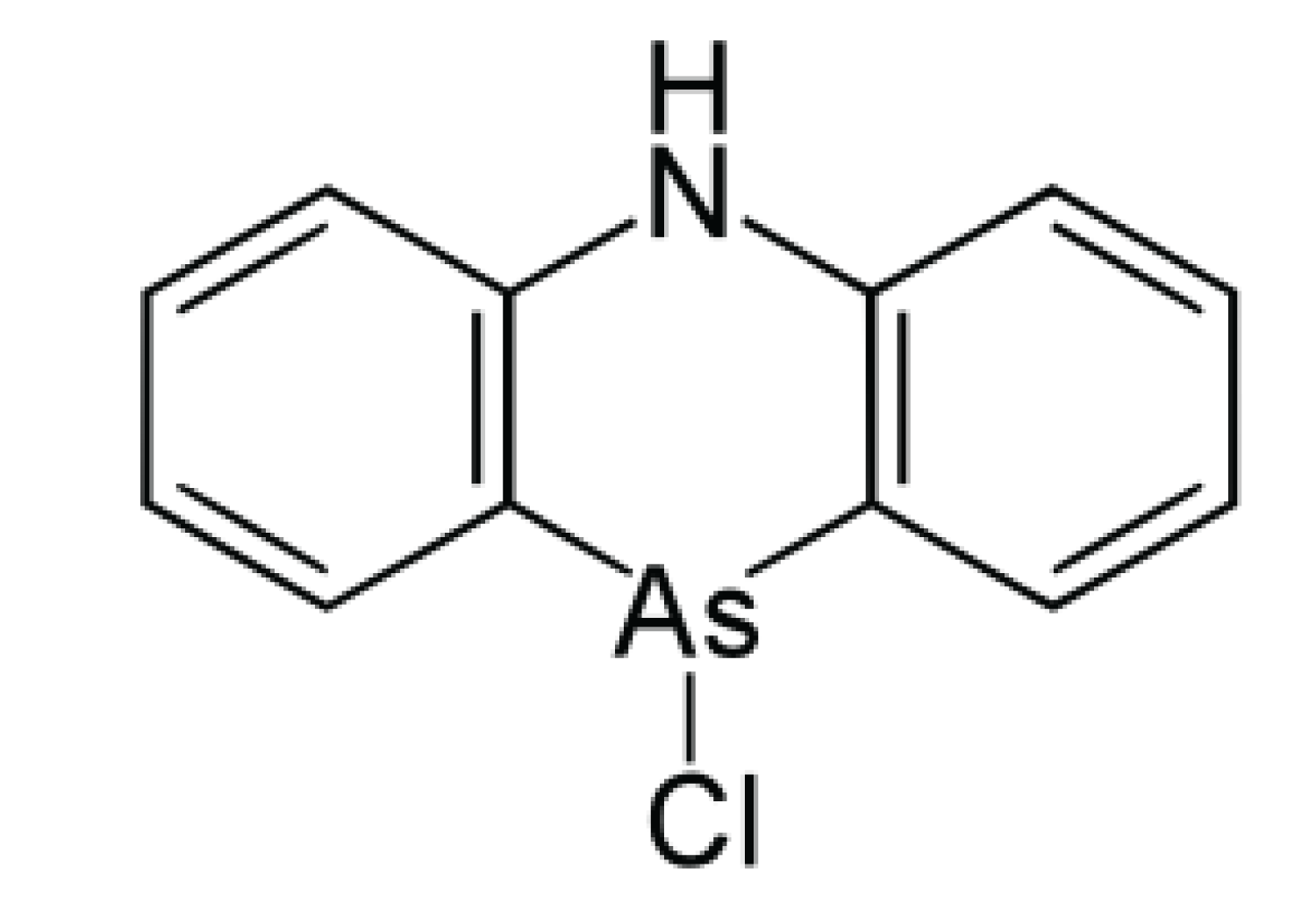
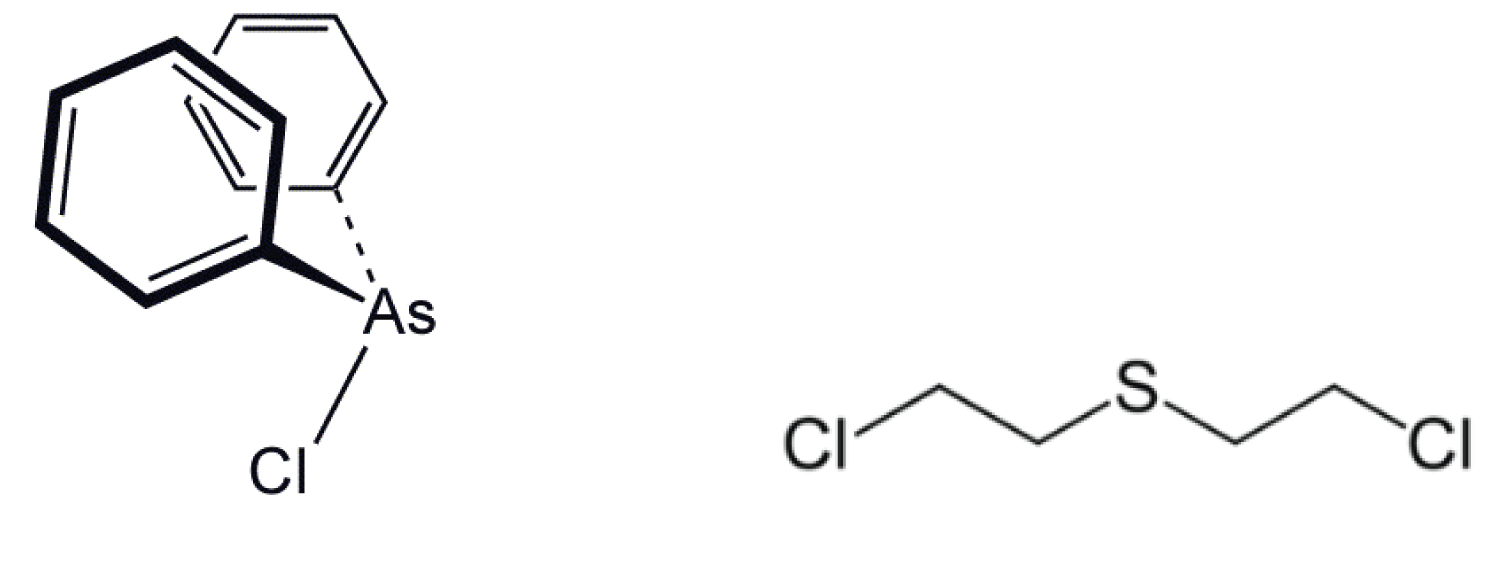
Adamsite [10-Chloro-5,10-dihydrophenazarsinine] or DM is an organic compound; technically, an arsenical diphenylaminechlorarsine, that can be used as a riot-control agent. DM belongs to the group of chemical warfare agents known as vomiting agents or sneezing gases. First synthesized in Germany by Heinrich Otto Wieland in 1915, it was independently developed by the US chemist Roger Adams (for whom it is named) at the University of Illinois in 1918.
Figure 13: Adamsite.
Figure 13: Adamsite.
DM was produced and stockpiled by the United States at the end of World War I, but not deployed on the battlefield then. It was used against the Bonus Army who demonstrated in Washington, DC, in 1932, reportedly causing the death and serious injury of several children who had accompanied their parents on the protests. It was again used in the Vietnam War. DM is an odorless crystalline compound with very low vapor pressure. The color of the crystals ranges from bright yellow to dark green depending on the purity. It is readily soluble in some organic solvents (e.g., acetone, dichloromethane), but nearly insoluble in water. In vaporous form, it appears as a canary yellow smoke. Adamsite is usually dispersed as an aerosol, making the upper respiratory tract the primary site of action. Although the effects are similar to those caused by typical riot control agents (e.g. CS), they are slower in onset and longer in duration, often lasting several hours. After a latency period of 5–10 minutes irritation of the eyes, lungs, and mucous membranes develops followed by headache, nausea, and persistent vomiting. DM is now regarded as obsolete. It has been widely replaced by riot control agents such as CS which are less toxic and more rapid in the onset of symptoms. Early battlefield use was intended to be via “Adamsite candles”. These were large metal cans or tubes (weighing approximately 5 pounds) which contained a smoke composition made of Adamsite plus a slow-burning pyrotechnic composition. A series of candles were lit and the Adamsite-laden smoke allowed to drift towards the enemy. In 2003, North Korea was reportedly producing Adamsite at its Aoji-RI Chemical Complex for stockpiling [23].
The sulfur mustards, or sulfur mustards, (ClCH2CH2SCH2CH2Cl) commonly known as mustard gas, are a class of related cytotoxic and vesicant chemical warfare agents with the ability to form large blisters on the exposed skin and in the lungs. Pure sulfur mustards are colorless, viscous liquids at room temperature. When used in an impure form, such as warfare agents, they are usually yellow-brown in color and have an odor resembling mustard plants, garlic, or horseradish, hence the name. Mustard gas was originally assigned the name LOST, after the scientists Wilhelm Lommel and Wilhelm Steinkopf, who developed a method for the large-scale production of mustard gas for the Imperial German Army in 1916. Mustard agents are regulated under the 1993 Chemical Weapons Convention (CWC). Three classes of chemicals are monitored under this Convention, with sulfur and nitrogen mustard grouped in Schedule 1, as substances with no use other than in chemical warfare. Mustard agents could be deployed on the battlefield by means of artillery shells, aerial bombs, rockets, or by spraying from warplanes. The compound readily eliminates a chloride ion by intramolecular nucleophilic substitution to form a cyclic sulfonium ion. This very reactive intermediate tends to permanently alkylate the guanine nucleotide in DNA strands, which prevents cell division and generally leads directly to programmed cell death, or, if cell death is not immediate, the damaged DNA may lead to the development of cancer. Oxidative stress would be another pathology involved in sulfur mustard toxicity. Sulfur mustard is not very soluble in water but is very soluble in fat, contributing to its rapid absorption into the skin. In the wider sense, compounds with the structural element BCH2CH2X, where X is any leaving group and B is a Lewis base is known as mustards. Such compounds can form cyclic “onium” ions (sulfonium, ammoniums, etc.) that are good alkylating agents. Examples are bis(2-chloroethyl)ether, the (2-haloethyl)amines (nitrogen mustards), and sulfur sesquimustard, which has two α-chloroethyl thioether groups (ClH2C-CH2-S-) connected by an ethylene (-CH2CH2-) group. These compounds have a similar ability to alkylate DNA, but their physical properties, e.g. melting point, vary [24].
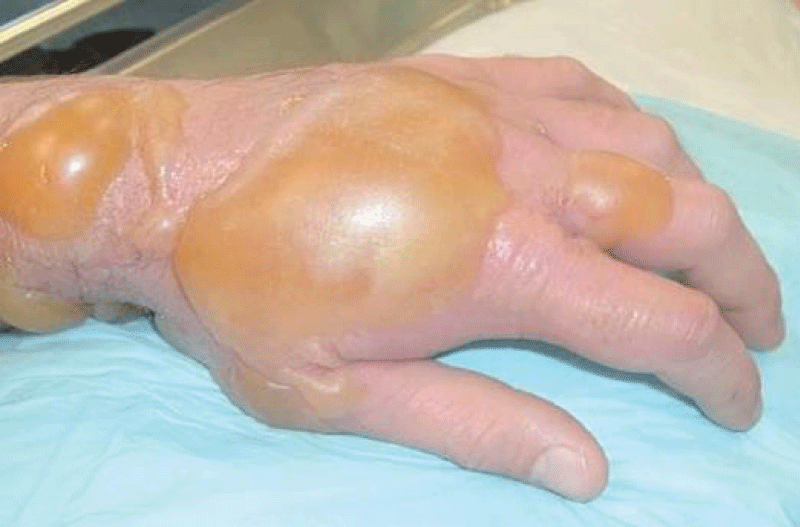
Figure 14: Toxic effect of sulfur mustard gas.
Figure 14: Toxic effect of sulfur mustard gas.
Mustard gas has extremely powerful vesicant effects on its victims. In addition, it is strongly mutagenic and carcinogenic, due to its alkylating properties. It is also lipophilic. Because people exposed to mustard gas rarely suffer immediate symptoms, and mustard-contaminated areas may appear completely normal, victims can unknowingly receive high dosages. Within 24 hours of exposure to the mustard agent, victims experience intense itching and skin irritation, which gradually turns into large blisters filled with yellow fluid wherever the mustard agent contacted the skin. These are chemical burns and are very debilitating. Mustard gas vapor easily penetrates clothing fabrics such as wool or cotton, so it is not only the exposed skin of victims that gets burned. If the victim’s eyes were exposed then they become sore, starting with conjunctivitis, after which the eyelids swell, resulting in temporary blindness. Miosis may also occur, which is probably the result of the cholinomimetic activity of mustard. At very high concentrations, if inhaled, the mustard agent causes bleeding and blistering within the respiratory system, damaging mucous membranes and causing pulmonary edema. Depending on the level of contamination, mustard gas burns can vary between first and second-degree burns, though they can also be every bit as severe, disfiguring, and dangerous as third-degree burns. Severe mustard gas burns (i.e. where more than 50% of the victim’s skin has been burned) are often fatal, with death occurring after some days or even weeks have passed. Mild or moderate exposure to mustard agents is unlikely to kill, though victims require lengthy periods of medical treatment and convalescence before recovery is complete. The mutagenic and carcinogenic effects of mustard agents mean that victims who recover from mustard gas burns have an increased risk of developing cancer in later life. Typical appearance of bullae on arm caused by blister agent burns. Skin damage can be reduced if povidone-iodine in a base of glycerol is rapidly applied, but since mustard agent initially has no symptoms, exposure is usually not recognized until skin irritation begins, at which point it is too late for countermeasures. The vesicant property of mustard gas can be neutralized by oxidation or chlorination, using household bleach (sodium hypochlorite), or by a nucleophilic attack using e.g. decontamination solution “DS2” (2% NaOH, 70% diethylenetriamine, 28% ethylene glycol monomethyl ether). After initial decontamination of the victim’s wounds is complete, medical treatment is similar to that required by any conventional burn. The amount of pain and discomfort suffered by the victim is comparable as well. Mustard gas burns heal slowly, and, as with other types of burns, there is a risk of sepsis caused by pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa. A British nurse treating soldiers with mustard gas burns during World War I commented: They cannot be bandaged or touched. We cover them with a tent of propped-up sheets. Gas burns must be agonizing because usually, the other cases do not complain, even with the worst wounds, but gas cases are invariably beyond endurance and they cannot help crying out [25].
Bis(chloromethyl) ether (ClCH2OCH2Cl) is carcinogenic. It is one of 13 chemicals considered an OSHA-regulated occupational carcinogen. Chronic exposure can increase the incidence of oat cell carcinoma, a type of lung cancer.
Ethyldichloroarsine, sometimes abbreviated “ED”, is an organoarsenic compound with the formula CH3CH2AsCl2. This colorless volatile liquid is a highly toxic obsolete vesicant or blister agent that was used during World War I in chemical warfare. The molecule is pyramidal with the Cl-As-Cl and C-As-Cl angles approaching 90°. Its toxic action is similar to lewisite. Blue Cross (Blaukreuz) is a World War I chemical warfare agent consisting of diphenylchlorarsine (DA, Clark I), diphenylcyanoarsine (CDA, Clark II), ethyldichloroarsine (Dick), and/or methyldichloroarsine (Methyldick). Clark, I, and Clark II were the main agents used. Clark I was used with Green Cross munition earlier; however, for the first time, it was used as a standalone agent in the night from July 10 to July 11 1917 at Nieuwpoort, Belgium, during the operation Strandfest. The artillery munition used as a delivery vehicle contained a large number of glass spheres closed with a cork and sealed with trinitrotoluene. Later N-ethyl carbazole was added. Depending on the caliber, the munition contained between 7 and 120 kilograms of the agent. Blue Cross is also a generic World War I German marking for artillery shells with chemical payload affecting the upper respiratory tract.
Figure 15: Diphenyl chloroarsine & Sulfur mustard.
Figure 15: Diphenyl chloroarsine & Sulfur mustard.
Green Cross (Grünkreuz) is a World War I chemical warfare pulmonary agent consisting of chloropicrin (PS, Aquinite, Klop), phosgene (CG, Collongite), and/or trichloromethyl chloroformate (Surpalite, Perstoff). Green Cross is also a generic World War I German marking for artillery shells with pulmonary agents (chemical payload affecting the lungs). The tip of the projectile with the fuse end is painted green and has a green cross at the bottom of the cartridge. Other Green Cross mixtures were based on phosgene and/or diphosgene. The first use of the Green Cross was on May 31st 1915 in a German offensive in Ypres. The mixture was chlorine-phosgene, with 95% and 5%.
Yellow Cross (Gelbkreuz) is a World War I chemical warfare agent usually based on sulfur mustard (HS, Yperite, Lost). The original Gelbkreuz was a composition of 80% - 90% of sulfur mustard and 10% - 20% of tetrachloromethane or chlorobenzene as a solvent which lowered its viscosity and acted as antifreeze, or, alternatively, 80% sulfur mustard, 10% bis(chloromethyl) ether, and 10% tetrachloromethane. A later formulation, Gelbkreuz 1, was a mixture of 40% ethyldichloroarsine, 40% ethyl-dibromo arsine, and 20% of bis(chloromethyl) ether. In some cases, nitrobenzene was used to mask the material’s characteristic odor. French “ypérite no.20” was a similar mixture of 80% sulfur mustard and 20% tetrachloromethane. Yellow Cross is also a generic World War I German marking for artillery shells with chemical payload affecting exposed surfaces of the body.
White Cross (Weisskreuz) is a World War I chemical warfare agent consisting of one or more lachrymatory agents: bromoacetone (BA), bromobenzyl cyanide (Camite), bromomethyl ethyl ketone (homomartonite, Bn-staff), chloroacetone (Tonite, A-staff), ethyl bromoacetate, and/or xylyl bromide. During World War I, White Cross was also a generic code name used by the German Army for artillery shells with an irritant chemical payload affecting the eyes and mucous membranes [25].
Figure 16: Detonator usage.
Figure 16: Detonator usage.
Investigation techniques: Explosive investigation is a difficult task as most of the evidence would have already been lost.
Process of investigation:
1. Initial response to information:
A. Date and Time
B. Brief details of damage
C. Number of people killed and injured
D. Whether anyone was seen carrying a bomb
E. Type of bag/vehicle, motorcycle, scooter
F. Address and name of the building, P.S., owner of the building
2. Approach to the scene:
A. First of all inform the bomb disposal squad
B. Take prevention of further explosion
C. To reveal the cause of the explosion
3. Secure the general area:
A. Vacate the place if it multi-storeyed building
B. Cordoning of the area. Generally, 300 meters radius from the site of the explosion
C. Classify the explosion: Major/Minor: Accidental/Terr.3orists Activities
D. Take Photographs/Video: Seat of Explosion
Location of fragments
Crater size
Injuries of victims
Dead bodies
4. Search Methods (Out Door)
A. Quadrant zone method
B. Strip Method
C. Grid Method
D. Spiral Method
E. Wheel Method
5. Don’ts:
1. Do not touch or remove the packet unless duty-bound.
2. Do not open the package with your hands.
3. Do not puncture the package.
4. Do not submerge the package in water.
5. Do not pull out the strings or wire.
6. Do not pass the metallic object over the package.
7. Handle the package alone.
8. Do not accept the identification marks on the package at the face value.
9. Do not bring a bomb or suspected object in a station house or inhabited building.
10. Do not use the radio in the vicinity of the bomb.
11. Evacuate the people to a safe distance. Always evacuate the people and NOT the BOMB.
12. Do not direct a flashlight on the bomb.
13. Remove all inflammable items.
14. Open windows and doors to minimize the blast effects.
15. Place sandbag around the object. Do not cover the object.
16. Do not permit re-entry of people until objects are removed.
17. Do not be a DEAD HERO. You can construct a building or house but you cannot make a dead man alive.
6. Actions on locating the bomb
1. Isolate the bomb
2. Remove essential/important items
3. Inform Bomb Squad
4. Inform Superior Officers
5. Protective Works like the use of sandbags/bomb blanket
6. Evacuate if not done earlier
7. Inform police/’Fire Service/Hospital
8. Post Guides
9. Assist Police/BD Squad
Collection: A most important step in the detection and analysis of explosive residues is the proper collection of appropriate samples from the explosion scene. Invariably undetonated residues or portions of the explosive remains at the site of the explosion. Forensic Laboratory analysis of these samples depends upon the I.O.’s ability and skill to recognize those samples and collection. The entire area must be systematically searched with great care, safety will be a major area of concern. The scene of the explosion is very unsafe, unexploded devices may still be in the area, the structure of a building where a bomb has exploded may be seriously weakened and can collapse. There may be additional hazards such as broken gas, mains, downed electrical lines, etc. If an unexploded bomb is found, it is necessary to call a bomb technician to render the device safe. The most obvious characteristic of a high explosive is the presence of a crater at the origin of the blast. Once the crater has been located, all loose soil and other debris must immediately be removed from the crater and preserved for lab analysis. Other good sources of explosive residues and objects are located near the origin of detonation. In pipe bomb explosion, particles of the explosive are frequently found adhering to the pipe or to the pipe threads.
Guidelines for collection and packing of physical evidence for examination: No live bomb or detonator is accepted by the laboratory. Post-blast residues containing soil and other loose materials are to be stored in metal containers or plastic bags. No live bomb or detonator is accepted by the laboratory. Control soil has to be collected, labeled, packed and sent separately to avoid cross-contamination. Control samples in case of post-blast cases are to be collected by measuring the distance from the seat of the explosion to the farthest missile and adding 50% of the distance to it.
Dispatch: Only by special messenger all sealed materials should be sent to the forensic laboratory with proper specimen seal, authority letter, etc. Don’t send materials by post or by goods train. In case of unexploded explosive materials, explosives and detonators should be packed separately. Send only a representative sample of the explosive and not the whole amount. Inform the station master that the explosives are being carried on the train. Don’t send any live bombs.
The Research and Analysis Wing (abbreviated R&AW) is the foreign intelligence agency of India. The agency’s primary function is gathering foreign intelligence, counter-terrorism, counter-proliferation, advising Indian policymakers, and advancing India’s foreign strategic interests. It is also involved in the security of India’s nuclear program. The Intelligence Bureau (IB) is India’s domestic internal security and counter-intelligence agency under the Ministry of Home Affairs. It was founded in 1887 as Central Special Branch and is reputed to be the oldest such organization in the world. Until 1968, it handled both domestic and foreign intelligence after which the Research and Analysis Wing was formed specifically for foreign intelligence following that IB was primarily assigned the role of domestic intelligence and internal security. Arvind Kumar, the current director of the IB, took over from Rajiv Jain on 26 June 2019.
Figure 17: Dog squad.
Figure 17: Dog squad.
List of Intelligence Agencies in India: A nation’s security is not only dependent on its armed forces but also on intelligence agency networks to deter internal as well as external threats for the country.
Here’s the list of intelligence agencies in India.
National Investigation Agency (NIA): Established: 2009, Headquarters: New Delhi, India. Chief: IPS Kuldeep Singh.
The National Investigation Agency (NIA) is India’s premier agency to counter terrorism under the aegis of the Ministry of Home Affairs (MHA). The agency investigates terror-related crimes in the country without any special permission from the states. It further probes attacks targeting Indian interests abroad. It came into existence with the enactment of the National Investigation Agency Act 2008 after the 26/11 Mumbai terror attacks. The agency maintains the NIA Most Wanted list.
Wildlife Crime Control Bureau (WCCB): Established: 2006, Headquarters: New Delhi, India. Chief: Tilotama Varma.
The Wildlife Crime Control Bureau (WCCB) deals with organized wildlife crime in India. The statutory agency is under the aegis of the Ministry of Environment, Forest, and Climate Change. The Parliamentary Standing Committee on Science and Technology, Environment and Forests strongly emphasized the creation of statutory authority under the Wild Life Protection Act, 1972. The Wild Life (Protection) Amendment Act, 2006 mentions enabling provisions for constituting the Bureau.
National Technical Research Organization (NTRO): Established: 2004, Headquarters: New Delhi, India. Chief: Anil Dhasmana
The National Technical Research Organization (NTRO) is a technical intelligence agency of India under the aegis of the Prime Minister’s Office. The agency is responsible for geospatial intelligence and satellite imagery and provides technical intelligence to other agencies on internal and external security. The agency was initially known as National Technical Facilities Organisation (NTFO) and was constituted upon the recommendations of the Group of Ministers (GOM) headed by then Deputy Prime Minister L K Advani. It has the same norms of conduct as the IB and R&AW.
Serious Fraud Investigation Office (SFIO): Established: 2003, Headquarters: New Delhi, India. Chief: Keshav Chandra.
The Serious Fraud Investigation Office (SFIO) is a statutory corporate fraud investigating agency in India. It is under the aegis of the Ministry of Corporate Affairs and conducts multi-disciplinary investigations of major corporate frauds. The organization has experts from the financial sector, capital market, accountancy, forensic audit, taxation, law, information technology, company law, customs, and investigation. The agency was set up on the recommendations of the Naresh Chandra Committee on corporate governance in the backdrop of stock market scams. The then Vajpayee Government constituted the Serious Fraud Investigation Office (SFIO) on 9 January 2003.
Defense Intelligence Agency (DIA): Established: 2002, Headquarters: New Delhi, India. Chief: Lt. Gen. KJS Dhillon.
Defense Intelligence Agency (DIA) provides and coordinates defense and military intelligence to the Indian Armed Forces. DIA is under the aegis of the Ministry of Defence. The head of the agency serves as the principal advisor on matters of intelligence to the Minister of Defence and the Chief of Defence Staff. The much-awaited intelligence agency coordinating the intelligence arms of three military services came into force after the Kargil War and was formally recommended by the Group of Ministers (GOM) headed by then Deputy Prime Minister L K Advani.
National Crime Record Bureau (NCRB): Established: 1986, Headquarters: New Delhi, India. Chief: IPS Ramphal Pawar.
The National Crime Record Bureau (NCRB), under the aegis of the Ministry of Home Affairs (MHA), deals in collecting and analyzing crime data as per the IPC and SLL. It functions as a repository of information on crime and criminals to help assist the investigators in linking crime to the perpetrators. The agency was set up on the recommendation of the Taskforce 1985, and the National Police Commission 1977 by merging the Directorate of Coordination and Police Computer (DCPC), Inter-State Criminals Data Branch of CBI, and Central Finger Print Bureau of CBI.
Narcotics Control Bureau (NCB): Established: 1986, Headquarters: New Delhi, India. Chief: IPS Satya Narayan Pradhan.
Narcotics Control Bureau (NCB) is the central law enforcement and intelligence agency that combats drug trafficking and the use of illegal substances as per the provisions of the NDPS Act. The agency further guides the State to exempt illegal substances for medicinal purposes.
The NCB functions through various zones and sub-zones. The zones are located in Ahmedabad, Bengaluru, Chandigarh, Chennai, Delhi, Guwahati, Indore, Jammu, Jodhpur, Kolkata, Lucknow, Mumbai, and Patna while the sub-zones are located in Ajmer, Amritsar, Bhubaneswar, Dehradun, Goa, Hyderabad, Imphal, Mandsaur, Madurai, Mandi, Raipur, Ranchi, and Kochi.
Research and Analysis Wing (R&AW): Established: 1968, Headquarters: New Delhi, India. Chief: Samant Goel. The Research and Analysis Wing (R&AW) is the external intelligence agency of India. The agency headed by the Prime Minister of India gathers foreign intelligence, counter-terrorism, counter-proliferation, advises Indian policymakers, and advances India’s foreign strategic interests. R&AW is also involved in the security of India’s nuclear program. R&AW came into being post the 1962 Sino-Indian War and the 1965 India-Pakistan War which exposed the foreign intelligence failures by the IB. The Indira Gandhi-led Central Government felt the need for a second security service in India and constituted the full-fledged Research and Analysis Wing (R&AW). The agency has been organized on the lines of the CIA.
Central Board of Indirect Taxes and Customs (CBIC): Established: 1944, Headquarters: New Delhi, India. Chief: IRS Vivek Johri.
The Central Board of Indirect Taxes and Customs (CBIC) is India’s nodal agency which is responsible for administering Customs, GST, Central Excise, Service Tax & Narcotics in India.
Central Bureau of Investigation (CBI): Established: 1942, Headquarters: New Delhi, India. Chief: Subodh Kumar Jaiswal.
India’s premier investigating agency, the Central Bureau of Investigation (CBI), operates under the jurisdiction of the Ministry of Personnel, Public Grievances, and Pensions. The agency is exempted from the provisions of the Right to Information (RTI) Act. It is India’s designated single point of contact for liaison with Interpol. The Central Bureau of Investigation (CBI) was originally constituted to investigate bribery and governmental corruption and was expanded in 1965 to investigate breaches of central laws enforceable by GOI, multi-state organized crime, multi-agency, or international cases.
Intelligence Bureau (IB): Established: 1887, Headquarters: New Delhi, India. Chief: Arvind Kumar.
Intelligence Bureau (IB) is the domestic internal security and counter-intelligence agency of India. The agency is under the aegis of the Ministry of Home Affairs (MHA). The agency was founded as Central Special Branch and was renamed after Indian Independence as Intelligence Bureau. It is reputed to be the oldest such organization in the world.
Dog Squad is a reality TV show following working dogs and handlers as they go about their daily duties. Dog Squad currently airs between 8:00 - 8:30 pm on Mondays on TV One. It was created by the same people who created Border Patrol. It is also termed as Explosive Detection Dog (EDD) or Explosive Detection Dog Team (EDDT). These are the K9s trained to detect explosive substances/presence of bomb/improvised explosive device (IED) in various operational environments and indicate/alert the handler when pressed into service.
Poison gas is any gas that is also a poison. Poison gases can kill or injure a person if present in a high enough concentration. There is a diverse range of different poison gases and each has unique properties. Many toxic liquids are also volatile and their vapors are poison gas. “Poison gas” can refer to poisons in chemical weapons, but most of them are in fact liquids, for example, mustard gas and VX are viscous liquids that are dispersed into fine mists. In small amounts, corrosive poison gases usually cause irritation and may have a smell, but this is not universal. There are several gases that can kill insidiously, without a warning smell or irritation. All gases other than oxygen can displace air and cause death by asphyxiation. This does not make them poisonous gases. Nitrogen and carbon dioxide are two common examples. This has to be taken into account in the handling of any compressed or liquified gas. Gases can be also dangerous because they are flammable (easily bursting into flame). Poison gases can cause injury more readily than condensed poisons because they diffuse (move) in the air, exposing skin, and are easily breathed in. Immediate contact with corrosive gas leads to chemical burns of the skin and lungs. When absorbed into the inside of the human body and into the blood, either by breathing in or through the skin, several poison gases cause poisoning. In a poisoning, medical help is often necessary; simply removing the victim from the gas is not enough to prevent symptoms. Corrosive poison gases such as hydrogen chloride cause chemical burns into the skin and inside the lungs. This causes the lungs to fill up with liquid, which can kill. Alkylating poison gases such as methyl chloride are attacked by human DNA and proteins. In human cells, this causes cell death, cancer, and a diverse set of symptoms caused by the malfunctioning of the alkylated proteins. Mustard gas is an alkylating agent. Gases that release the fluoride ion into the human body can cause a loss of calcium in the blood, which leads to heart attack and death. Examples are hydrogen fluoride and chlorine trifluoride. Ammonia can cause corrosive burns. Hydrogen sulfide has a powerful smell, but it will easily desensitize the nose. After that, it can shut down the breathing center in the brain, which stops the breathing reflex and kills the victim from asphyxiation. Nerve agents are poisonous liquids that easily evaporate. They shut down biochemical processes that allow muscles to relax. The result is that all muscles contract, including muscles required for breathing, causing death by asphyxiation. Poison gases are used in industry as chemical reagents. The chemical reactions they can be used for are more important than their toxicity. Today, chemists try to avoid the use of poisonous gases, but it is often not possible. Examples of large-volume industrial poisonous gases are hydrogen sulfide, cracked from oil, chlorine, in diverse chemical uses and to disinfect drinking water, and ammonia, which is a valuable fertilizer into itself and can be converted into a range of other fertilizers, in addition to chemical uses. Some fumigants (chemicals used to kill pests) are also poison gases, for example, phosphine, which is used to kill pests living in grains. The advantage of a gas is that it evaporates after use so that if food is treated with gas, there is no need to rinse it to remove the poison.
- Patel VR, Parmar JN, Aal LB, Sen DJ. Poison gas: as a lethal weapon in chemical warfare. Int J Pharmaceut Res Bio-Sci. 2014; 3: 332-357.
- Krehl POK. History of Shock Waves, Explosions and Impact: A Chronological and Biographical Reference. Springer Science & Business Media. 1970; 2008.
- Borak J, Sidell FR. Agents of chemical warfare: Sulfur mustard. Ann Emerg Med. 1992; 21: 303–308. PubMed: https://pubmed.ncbi.nlm.nih.gov/1536492/
- Cooper, Paul W. Chapter 4: Use forms of explosives. Explosives Engineering. Wiley-VCH. 1996; 51–66.
- Assovskiy IG. Direct laser initiation of open secondary explosives. J Phys. 2015; 653: 012-014.
- Ellison DH. Handbook of Chemical and Biological Warfare Agents, Second Edition. CRC Press. 2007; 456.
- Gao Y, Olomon PM. HCN Survey of Normal Spiral, Infrared‐luminous, and Ultraluminous Galaxies. Astrophysical J Suppl Series. 2004; 152: 63–80.
- Borowitz JL, Gunasekar PG, Isom GE. Hydrogen cyanide generation by mu-opiate receptor activation: possible neuromodulatory role of endogenous cyanide. Brain Res. 1997; 768: 294–300. PubMed: https://pubmed.ncbi.nlm.nih.gov/9369328/
- Hu H, Fine J, Epstein P, Kelsey K, Reynolds P, et al. Tear gas--harassing agent or toxic chemical weapon? JAMA. 1989; 262: 660–663. PubMed: https://pubmed.ncbi.nlm.nih.gov/2501523/
- Levin BC, Paabo M, Gurman JL, Harris SE, Braun E. Toxicological interactions between carbon monoxide and carbon dioxide. Toxicology. 1987; 47: 135–164. PubMed: https://pubmed.ncbi.nlm.nih.gov/3120355/
- Dabrowska MI, Becks LL, Lelli JL, Jr, Levee MG, Hinshaw DB. Sulfur Mustard Induces Apoptosis and Necrosis in Endothelial Cells. Toxicol Appl Pharmacol. 1996; 141: 568–583. PubMed: https://pubmed.ncbi.nlm.nih.gov/8975783/
- Abu-Qare AW, Abou-Donia MB. Sarin: health effects, metabolism, and methods of analysis. Food Chem Toxicol. 2002; 40: 1327–1333. PubMed: https://pubmed.ncbi.nlm.nih.gov/12387297/
- Kovarik Z, Radić Z, Berman HA, Simeon-Rudolf V, Reiner E, et al. Acetylcholinesterase active centre and gorge conformations analysed by combinatorial mutations and enantiomeric phosphonates. Biochem J. 2003; 373: 33–40. https://pubmed.ncbi.nlm.nih.gov/12665427/
- Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, et al. Crystal Structures of Aged Phosphonylated Acetylcholinesterase: Nerve Agent Reaction Products at the Atomic Level. Biochemistry. 1999; 38: 7032–7039. https://pubmed.ncbi.nlm.nih.gov/10353814/
- Petroianu G. Pharmacists Adolf Schall and Ernst Ratzlaff and the synthesis of tabun-like compounds: a brief history. Die Pharmazie. 2014; 69: 780–784. PubMed: https://pubmed.ncbi.nlm.nih.gov/25985570/
- Jokanović M. Biotransformation of organophosphorus compounds. Toxicology. 2001; 166: 139–160. PubMed: https://pubmed.ncbi.nlm.nih.gov/11543910/
- Cowell EM. Chemical Warfare and the Doctor. Bri Med J. 1939; 2: 736-738. PubMed: https://pubmed.ncbi.nlm.nih.gov/20782694/
- Fieldner AC, Katz SH, Kinney SP, Longfellow ES. Poisonous gases from carbon tetrachloride fire extinguishers. J Franklin Institute. 1920; 543–565.
- Wagner G. Sitzung der russischen chemischen Gesellschaft am 7/19. Berichte de Deutschen Chemischen Gesellschaft. 1876; 9: 1687–1688.
- Snyder LE, Buhl D. Observations of Radio Emission from Interstellar Hydrogen Cyanide. Astrophysical J. 1971; 163: L47–L52.
- Ramasamy S, Singh S, Taniere P, Langman MJS, Eggo MC. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am J Physiol. 2006; 291: G288–G296. PubMed: https://pubmed.ncbi.nlm.nih.gov/16500920/
- Piantadosi CA. Carbon monoxide poisoning. Undersea Hyperb Med. 2004; 31: 167–177. PubMed: https://pubmed.ncbi.nlm.nih.gov/15233173/
- Bunn G. Banning Poison Gas and Germ Warfare: Should the United States Agree. Wisconsin Law Rev. 1969; 2: 405.
- Duchovic RJ, Vilensky JA. Mustard Gas: Its Pre-World War I History. J Chem Educ. 2007; 84: 944.
- Heitmann W, Strehlke G, Ethers DM, Aliphatic Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002