Research Article
Organic compositional analysis of propellant powders using monolithic material sorption extraction (MSSE)-a feasibility study

Ellen Goudsmits, George P Sharples and Jason W Birkett*
School of Pharmacy and Biomolecular Sciences, Faculty of Science, Liverpool John Moores University, Byrom Street, Liverpool, L3 3AF, UK
*Address for Correspondence: Jason W Birkett, School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, James Parsons Building, Byrom Street, Liverpool, L3 3AF, UK, Tel: +44 0151 231 2041, Fax: +44 0151 231 2170; E-mail: [email protected]
Dates: Submitted: 22 August 2017; Approved: 01 September 2017; Published: 05 September 2017
How to cite this article: Goudsmits E, Sharples GP, Birkett JW. Organic compositional analysis of propellant powders using monolithic material sorption extraction (MSSE)-a feasibility study. J Forensic Sci Res. 2017; 1: 068-076.
DOI: 10.29328/journal.jfsr.1001008
Copyright License: © 2017 Goudsmits E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Forensic science; Ballistics; Propellant; MonoTrap; Chromatography
Abstract
The application of monolithic material sorption extraction, specifically MonoTrapTM, to the extraction of organic gunshot residue (OGSR) compounds from unburnt propellant powders is described. Four different MonoTraps were assessed for their capability to extract OGSR compounds from two different ammunition types. Extracts were analysed using gas chromatography-mass spectrometry. Results indicated that the carbon disc was suitable for the extraction of OGSR compounds from unburnt propellant powders. Quantities for major compounds were comparable to methanol extractions. The method was successfully used to detect a wide range of OGSR compounds, including nitrotoluenes, nitroglycerin, diphenylamines and centralites and is expected to be applicable to a wide range of ammunition types.
Introduction
The analysis of additives in smokeless propellant powder is often an integral part of investigations of improvised explosive devices (IEDs) and the evaluation of organic gunshot residue (OGSR) [1,2]. Recently, several methods for the characterisation of OGSR compounds in smokeless propellant powders have been developed [3-5]. Both solvent extraction and solid-phase microextraction (SPME) have been employed for this purpose.
Monolithic material, which is used for sorptive extraction, consists of rigid macroporous polymers prepared by bulk polymerisation in a closed mould [6]. The material is created by polymerisation of a monomer mixture with a porogenic solvent, forming a highly porous polymer bed [7]. Inorganic monolithic material, which is used for MonoTrapTM, mainly consists of porous high-purity silica [6,8]. Monolithic material is also widely used as a stationary phase in liquid chromatography [7,9-11]. Other applications include its use as a column for anion-exchange chromatography [12], in tube solid-phase extraction (SPE) [9], in needle extraction [10,13] and stir bar/stir rod sorptive extraction (SBSE/SRSE) [7,14,15]. MonoTraps are employed for a wide range of biological applications, including the extraction of flavour and aroma compounds [16], the characterisation of odorants [17], and the determination of plant hormones [18]. It has wide uses in separation science and in (bio) catalysis [9], and is used for sample preparation in drug and pharmaceutical analysis [11].
MonoTrapTM has a large surface area (150 m2/gram or more), which is provided by the network of interconnected pores in the silica frame [7,8,19]. Due to the network of pores, with sizes in the low micrometer range, this material possesses very good permeability. Consequently, high analyte migration rates are achieved [7,8,11,19]. This results in significantly faster extraction times for stir bars coated with monolithic materials (up to a few hours [7,14]) than for stir bars coated with a thick layer of a material, such as polydimethylsiloxane (PDMS) (up to 72 hours [20]). MonotrapTM is suitable for non-polar to polar compounds [19] and it requires only 200 µL of solvent to perform the extraction, which is both environmentally and economically advantageous [4]. MonoTrapTM is available in a silica type and in a type in which the silica frame contains activated carbon that acts as an adsorptive medium. This type of MonoTrapTM has octadecyl-groups conjugated to its frame [8,11]. Both the silica and the carbon type are available in disc and rod configurations [11].
The application of monolithic material sorption extraction (MMSE), more specifically MonoTrapTM, to the extraction of OGSR compounds commonly found in smokeless propellant powders and GSR samples is examined for the first time in this study. The ability to extract a wide range of OGSR compounds is demonstrated using a standard solution. The selection of the compounds included in the standard is based on previous work, in which relevant compounds associated with propellant are highlighted [4,21]. A comprehensive list of organic compounds associated with propellant and GSR can be found elsewhere [22]. The capability of MonoTrapTM to extract OGSR compounds from unburnt ammunition propellant is evaluated. Different types (i.e. silica and carbon) and different configurations (i.e. discs and rods) are compared, and the ability of these materials to recover OGSR compounds present in propellant powder, and thus potentially in OGSR samples, is discussed.
Materials and Methods
Materials and Methods
Solvents and standards
Camphor, carbazole, diphenylamine (DPA), 4-nitrodiphenylamine (4-NDPA), 2,4-dinitrodiphenylamine (2,4-DNDPA), ethylphenylamine (EPA), dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP), diisobutyl phthalate (DIBP), 3-nitrotoluene (3-NT), 2,3-dinitrotoluene (2,3-DNT), 2,4dinitrotolune (2,4-DNT), 2,6-dinitrotoluene (2,6-DNT), 3,4-dinitrotoluene (3,4-DNT), and triacetin were purchased from Sigma Aldrich (Bellefonte, PA, USA). 2-Nitrodiphenylamine (2-NDPA) was obtained from LGC Standards (Middlesex, UK). Methyl centralite (MC) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Analytical grade methanol (Sigma Aldrich, UK) was used for the preparation of the standard. MonoTrap desorption was performed with analytical grade methanol or dichloromethane (DCM) (Fisher Scientific, Loughborough, UK).
Acquisition of propellant
Hodgdon HP-38 propellant for self-loading ammunition was obtained from the Wellington Rifle and Pistol Club (Skipton, Yorkshire, UK). Nottinghamshire police provided. 223 Magtech Rem tactical rounds, of which the bullet was pulled using a kinetic hammer in order to collect the unburnt propellant.
MonoTrapTM extractions
MonoTrapTM adsorptives (silica disc, silica rod, carbon disc, and carbon rod) were purchased from GL Science Inc. (Tokyo, Japan). The extractions were carried out by placing the MonoTrapTM directly onto 100 mg propellant powder or onto the standard solution. The vial was then placed in an oven (Nabertherm) at 80°C for 3 hours as per the manufacturer’s recommendations.
Analyte desorption was accomplished by solvent extraction of the MonoTrapTM with 200 µL of methanol or DCM. After 5 minutes of sonication at ambient temperature, the MonoTrapTM was removed and as much of the solvent as possible was transferred to a GC vial (Chromacol ltd., Herts, UK) containing a 150 µL glass insert with polymeric feet (Agilent Technologies, Santa Clara, CA, USA) [8]. Sequential desorption of a single MonoTrapTM was also attempted by performing the desorption procedure with DCM, and subsequently repeating it using methanol.
Methanol extractions of propellant powder
Methanol extractions were performed by adding 2 mL of methanol to 100 mg propellant powder. The vials were sonicated for 1 hour [4], after which the supernatant was centrifuged for 15 minutes and then filtered through a 45 µm PTFE filter.
GC-MS instrumentation and conditions
Chromatographic analysis, optimised from the method reported by Dalby & Birkett [4], was performed on an Agilent 6890N Network GC system, equipped with a J&W Scientific HP5-MS UI (30m x 0.25 mm x 0.25 µm) column. Sample introduction was performed using an autosampler (Agilent 7683 series). Splitless injection and a solvent delay of 1.8 minutes were used for all samples. The following chromatographic conditions were used: initial oven temperature of 50°C, rising by 10°C/min to 100°C, second ramp of 5°C/min to 180°C and held for 2.5 min, third ramp of 30°C/min to 200°C and held for 2.5 min, and a fourth ramp of 30°C/min to 300°C, which was held for 2 min. The total run time was 32 minutes. The flowrate of the carrier gas (helium) was maintained at 1.2 mL/min. Methanol blanks were run in between samples. Statistical processing of the data was performed on the obtained peak areas.
The GC was coupled with an Agilent 5975B Inert MSD system using electron ionisation (EI). In full scan mode, masses were scanned from m/z 40 to 500. Mass spectra for recorded peaks were further evaluated using the NIST database (MS search programme Version 2.0, NIST, MSS ltd. Manchester, England).
Results and Discussion
Extraction and separation of OGSR compounds
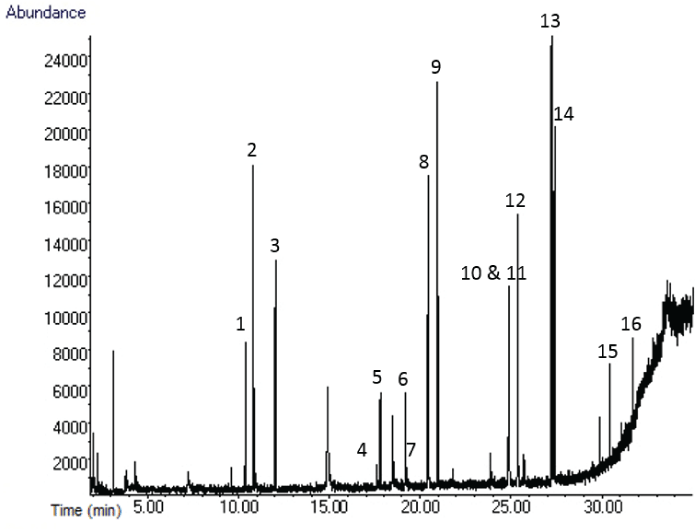
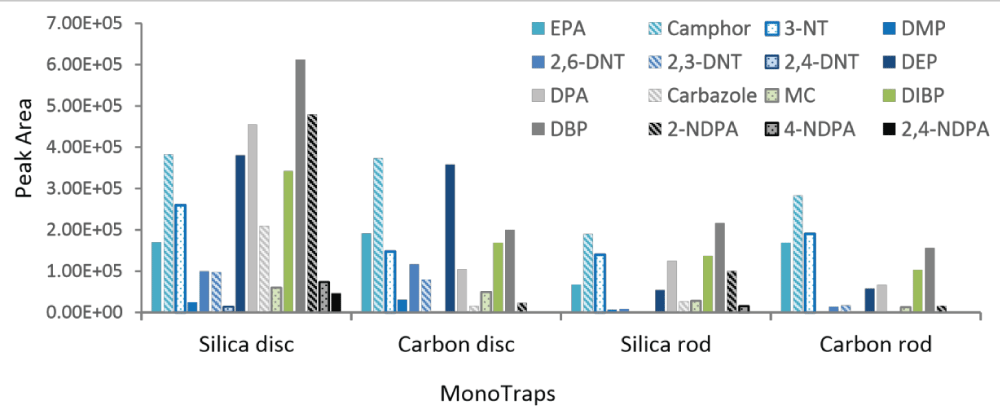
The ability of the MonoTraps to extract a wide range of OGSR compounds was demonstrated by the extraction of a standard solution. Good chromatographic separation was achieved for most compounds (Figure 1). Only the dinitrotoluenes (4 and 5, and 6 and 7) are not baseline resolved, and carbazole (10) and methyl centralite (11) co-eluted. All compounds, however, could be identified using mass spectral data. A comparison of the results of all four MonoTrapTM types is shown in figure 2.
Figure 1: Chromatogram silica disc extract of standard 1: 1. EPA, 2. Camphor, 3. 3-NT, 4. DMP, 5.2,6-DNT, 6. 2,3-DNT, 7. 2,4-DNT, 8. DEP, 9. DPA, 10. Carbazole, 11. MC, 12. DIBP, 13. DBP, 14. 2-NDPA, 15. 4NDPA, 16. 2,4-DNDPA.
The discs produced a higher peak area than the rods for all OGSR compounds, except for DBP that resulted in a greater peak area in the extract of the silica rod than carbon disc. This could be due to the limited surface contact the rods had with the sample; less than a quarter of the rod was in direct contact with the liquid standard during extraction, compared to half the disc. Submerging the rod to increase the surface area in contact with the sample resulted in very poor extraction, as indicated by the manufacturer’s information [8]. Both discs produced comparable peak areas for the first eight compounds shown in.
Figure 2, the latter eight compounds clearly produced higher peak areas using the silica disc. Despite the fact that the extraction using discs resulted in greater quantities detected than the rods for almost every compound, the silica MonoTraps extracted more compounds (a total of 16 and 14 compounds for the disc and rod respectively) than the carbon MonoTraps (a total of 13 and 11 compounds for the disc and rod respectively). These results demonstrate that the carbon rod is the least effective overall. The silica rod produced comparable values to the carbon rod but it enabled the extraction of more compounds than both of the carbon MonoTraps. The most effective extraction was achieved with the silica disc.
Propellant powder analysis
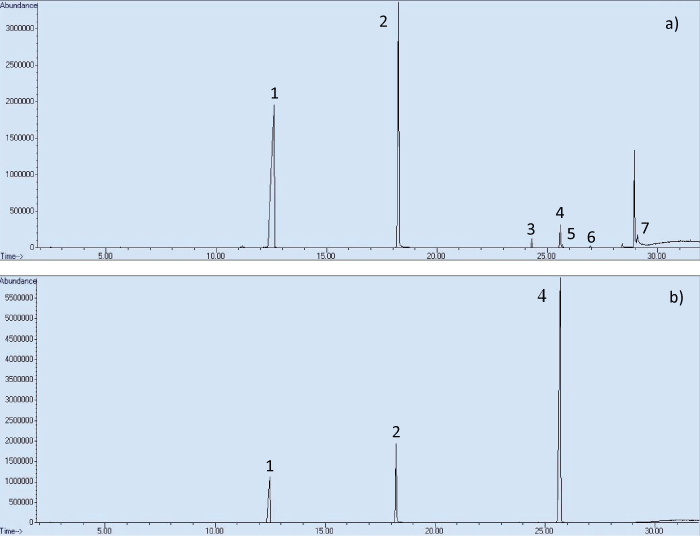
Methanol extractions: Figure 3 shows the chromatograms of the methanol extractions of the Hodgdon HP-38 (Figure 3a) and Magtech .223 (Figure 3b) propellant. The chromatogram of Hodgdon HP-38 shows all known OGSR compounds detected. Due to a greater difference in obtained peak areas, only the three major compounds are visible in the chromatogram of the Magtech propellant. The full list of compounds detected in both propellants, including the standard deviations of the average peak areas are shown in table 1.
Figure 3: Methanol extractions of 100 mg propellant: a) Hodgdon HP-38, b) Magtech .223 (1. NG, 2. DPA, 3. EC, 4. DBP, 5. 2-NDPA, 6. AKII, 7. 4-NDPA).
| Table 1: Mean peak areas (PA) and standard deviations for methanol extractions of two propellant powders | |||||
| Compounds | RTs (min) | Hodgdon HP-38 Mean PA (n = 3) Std dev (%) |
Magtech .223 Mean PA (n = 3) Std dev (%) |
||
| NG | 12.6 | 1.96E+08 | 4.46 | 5.99E+07 | 34.61 |
| DPA | 18.3 | 1.15E+08 | 3.27 | 5.40E+07 | 26.49 |
| DIBP | 23.8 | - | - | 3.02E+05 | 35.99 |
| EC | 24.3 | 3.01E+06 | 5.63 | 1.53E+05 | 34.73 |
| DBP | 25.6 | 9.06E+06 | 4.11 | 2.83E+08 | 24.34 |
| 2-NDPA | 25.7 | 1.95E+06 | 26.56 | - | - |
| AKII | 27.0 | 8.52E+05 | 8.46 | - | - |
| 4-NDPA | 29.1 | 3.16E+06 | 6.35 | 4.74E+05 | 13.14 |
| DPF | 21.8 | 1.14E+05 | 31.58 | 4.03E+04 | 42.00 |
The results show that both propellant powders have a different composition as a different combination of OGSR compounds has been detected, with varying relative abundances between the compounds. Good qualitative repeatability was obtained for each propellant, and a good chromatographic separation was observed for all detected compounds. Furthermore, low standard deviations were obtained for Hodgdon HP-38 propellant samples in general, however, the values for the Magtech propellant were lower and resulted in relatively high standard deviations. This is possibly due to the fact that the Magtech propellant appears to have a coating, which might inhibit the release of OGSR compounds [4].
Four compounds were detected in addition to the OGSR compounds that were included in the standard, namely nitroglycerin (NG) and ethyl centralite (EC) (both known OGSR compounds), akardite II (AKII) and N,N-diphenylformamide (DPF). AKII is a stabiliser that may be used as an additive in propellant powder and is thus also associated with OGSR materials [21-25]. DPF has only been mentioned in conjunction with OGSR in the literature on a few occasions, but its function in propellant powder was not reported [26,27].
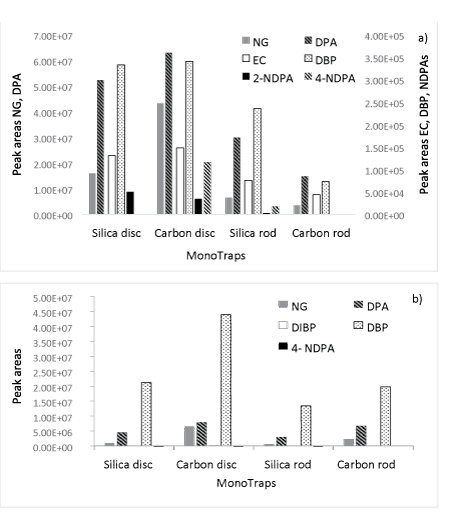
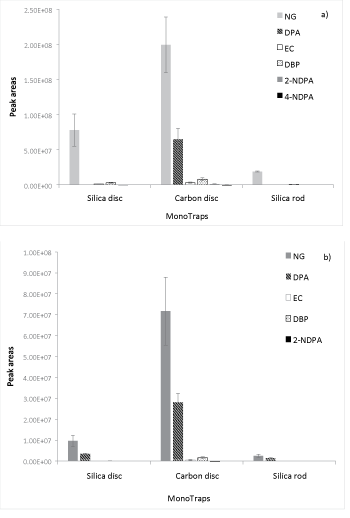
MMSE extraction of propellant extracts: Figure 4 shows the results of the extraction of Hodgdon HP-38 and Magtech .223 propellants.
Figure 4: Extractions of 100 mg propellant powder using MonotrapTM. a) Hodgdon HP-38 propellant, b) Magtech .223 propellant.
The MonoTrapTM extractions produced similar results to the methanol extractions of the propellant powders, particularly for the major compounds in each powder. DPA and NG respectively were the major compounds extracted from Hodgdon HP-38 propellant. Their quantities were much higher than the amounts of the other four OGSR compounds present (Figure 4a), which were all extracted using the carbon disc and to a lesser degree with the silica rod. In contrast to the results obtained from the standard extraction, the silica disc extracted one compound, 4-NDPA, less than the carbon disc. The only OGSR compound that was not extracted using the MonoTraps, was AKII.
NG, DPA and DBP were clearly present as major compounds in the Magtech propellant (Figure 4b), and similar peak areas were obtained. The achieved peak areas of DIBP and 4-NDPA varied according to MonoTrapTM type, and 4-NDPA was not detected in the carbon rod extracts. EC was the only compound that was not detected. The overall amounts extracted from the Magtech propellant were lower for all MonoTrapTM types, which could be due to the coating of the propellant grains [4].
For both propellant powders, the most effective extraction was achieved using the carbon disc. When comparing the results achieved with the extraction of the carbon disc against the methanol extractions of propellant powders, it shows that the average peak areas obtained per compound are about one order of magnitude lower for the carbon disc. Despite this fact, only two compounds, one per powder, were not detected using this method. It should be noted that AKII was not included in the standard and thus it is unknown whether this compound is suitable for extraction with the developed MMSE method. EC may not have been detected in the .223 Magtech propellant extracts as a result of the coating these powder grains appear to have. Lower overall yields were detected for the Magtech propellant compared to the Hodgdon propellant for both methanol extractions as well as MMSE.
MMSE extraction of propellant-DCM desorption: An initial attempt to improve the detection of these compounds was made by using DCM for the desorption of the MonoTraps following extraction of the Hodgdon propellant. This powder was selected as it contained the most OGSR compounds. The carbon rod was not tested due to its poor performance.
Desorption with DCM resulted in greater quantities detected for almost all compounds (Figure 5a). Subsequent methanol desorption, however, still resulted in relatively high yields of OGSR compounds (Figure 5b). Despite higher yields detected after DCM desorption, 4-NDPA was only detected in the carbon disc.
Figure 5: Extractions of 100 mg Hodgdon HP-38 propellant: a) DCM desorption, b) subsequent methanol desorption.
The improved desorption efficiency achieved with DCM resulted in the detection of peak areas of NG and EC obtained with the carbon disc that are comparable to the methanol extraction (Table 2). When combining the peak areas of the DCM and methanol fractions, the amounts for DPA and DBP are also comparable. The peak area values for 2-NDPA are approximately half of those produced by the methanol extraction, and only 4-NDPA remains one order of magnitude below the methanol extraction value. These results suggest that the extraction of propellant powder using the carbon disc and sequential desorption is a viable nearly solvent-free alternative to methanol extraction in the analysis of the major propellant compounds and even some of the minor compounds. Further optimisation of the desorption method is ongoing and could potentially lead to improvement beyond that obtained for methanol extractions, given the fact that NG and EC already resulted in slightly better yields.
| Table 2: Peak area comparison of various extraction methods for 100 mg Hodgdon HP-38 | ||||
Methanol |
Carbon disc |
Carbon disc |
Carbon disc extraction (methanol) |
(DCM) (methanol after DCM) |
NG |
1.96E+08 |
4.33E+07 |
1.99E+08 |
7.17E+07 |
DPA |
1.15E+08 |
6.29E+07 |
6.46E+07 |
2.81E+07 |
EC |
3.01E+06 |
1.48E+05 |
3.24E+06 |
5.17E+05 |
DBP |
9.06E+06 |
3.41E+05 |
8.04E+06 |
1.66E+06 |
2-NDPA |
1.95E+06 |
3.45E+04 |
1.02E+06 |
5.47E+04 |
AKII |
8.52E+05 |
0.00E+00 |
0.00E+00 |
0.00E+00 |
4-NDPA |
3.16E+06 |
1.16E+05 |
2.12E+05 |
0.00E+00 |
Conclusion
A method for the extraction of OGSR compounds from propellant powders, which enabled the effective separation and detection of a wide range of known OGSR compounds, has been presented. The major advantage of this method is that it requires only 200 µL of solvent to perform the extraction, which is both environmentally and economically advantageous. The results have clearly demonstrated that extraction of propellant powder using the carbon disc is a viable alternative to methanol extraction. Similar amounts were detected for the major propellant compounds using both methods. Minor OGSR compounds were also extracted and it is suspected that further optimisation of the desorption method would significantly improve their detection. The results from the optimisation work have shown that multiple solvents may be required to desorb all compounds.
Further work by the authors is focussing on the improvement of the desorption method in order to ensure that all extracted compounds are recovered from the MonoTrap materials. Due to its capability to extract a wide range of OGSR compounds, including nitrotoluenes, diphenylamines and centralites, this method is expected to be applicable to a much wider range of ammunition types than was initially tested here.
Acknowledgements
The authors would like to thank the School of Pharmacy and Biomolecular Sciences at Liverpool John Moores University for research funding through the Faculty of Science Ph.D Studentship Scheme.
References
- MacCrehan WA, Bedner M. Development of a smokeless powder reference material for propellant and explosives analysis. Forensic Sci Int. 2006; 163: 119-124. Ref: https://goo.gl/2FfnS9
- West C, Baron G, Minet JJ. Detection of gunpowder stabilizers with ion mobility spectrometry. Forensic Sci Int. 2007; 166: 91-101. Ref: https://goo.gl/rV78qb
- Thomas JL, Lincoln D, McCord BR. Separation and detection of smokeless powder additives by ultra-performance liquid chromatography with tandem mass spectrometry (UPLC/MS/MS). J Forensic Sci. 2013; 58: 609-615. Ref: https://goo.gl/gFeA8U
- Dalby O, Birkett JW. The evaluation of solid phase micro-extraction fibre types for the analysis of organic components in unburned propellant powders. J Chromatogr A. 2010; 1217: 7183-1788. Ref: https://goo.gl/W6p3oL
- Joshi M, Rigsby K, Almirall JR. Analysis of the headspace composition of smokeless powders using GC-MS, GC-µECD and ion mobility spectrometry. Forensic Sci Int. 2011; 208: 29-36. Ref: https://goo.gl/UX4m2g
- Svec F, Tennikova TB. Historical Review, in: Svec F, Tennikova TB, Deyl Z. (Eds.) Monolithic materials: preparation, properties and applications. Elsevier Science. 2003; 1-15.
- Huang X, Yuan D. Preparation of stir bars for sorptive extraction based on monolithic material. J Chromatogr A. 2007; 1154: 152-157. Ref: https://goo.gl/sr4gtZ
- GL Sciences Inc, MonoTrapTM guide to proper use, GL Sciences Inc. 2007; 1-36.
- Buchmeiser MR. Polymeric monolithic materials: Syntheses, properties, functionalization and applications. Polymer. 2007; 48: 2187-2198. Ref: https://goo.gl/2AUew3
- Pietrzynska M, Voelkel A, Bielicka-Daszkiewicz K. Preparation and examination of monolithic inneedle extraction (MINE) device for the direct analysis of liquid samples. Anal Chim Acta. 2013; 776: 50-56. Ref: https://goo.gl/aZnDDr
- Namera A, Saito T. Advances in monolithic materials for sample preparation in drug and pharmaceutical analysis. Trends in Analytical Chemistry. 2013; 45: 182-196. Ref: https://goo.gl/pz1TXm
- Gu H, Yin D, Ren J, Zhang B, Zhang Q. Preparation of quaternary amine monolithic column for strong anion-exchange chromatography and its application to the separation of Enterovirus 71. J Chromatogr B. 2016; 399-405. Ref: https://goo.gl/P3svgk
- Pietrzynska M, Tomczak R, Jezierska K, Voelkel A, Jampilek J. Polymer-ceramic Monolithic InNeedle Extraction (MINE) device: Preparation and examination of drug affinity. Mater Sci Eng C. 2016; 68: 70-77. Ref: https://goo.gl/Ea9h3y
- Luo YB, Ma Q, Feng YQ. Stir rod sorptive extraction with monolithic polymer as coating and its application to the analysis of fluoroquinolones in honey sample. J Chromatogr A. 2010; 1217: 3583-3589. Ref: https://goo.gl/BWWGfM
- Luo YB, Zheng HB, Wang JX, Gao Q, Yu QW, et al. An anionic exchange stir rod sorptive extraction based on monolithic material for the extraction of non-steroidal anti-inflammatory drugs in environmental aqueous samples. Talanta. 2011; 86: 103-108. Ref: https://goo.gl/GivpBh
- Wang S, He Y, Wang Y, Tao N, Wu X, et al. Comparison of flavour qualities of three sourced Eriocheir sinensis. Food Chem. 2016; 200: 24-31. Ref: https://goo.gl/NJ3xbs
- Zhao Lm, Wu W, Tao Np, Li Yq, Wu N, et al. Characterization of important odorants in four steamed Coilia ectenes from China by gas chromatography–mass spectrometry-olfactometry. Fisheries Science, 2015; 81: 947-957. Ref: https://goo.gl/CQUQEs
- Ma W, Fu S, Hashi Y, Chen Z. Determination of chiral jasmonates in flowers by GC/MS after monolithic material sorptive extraction. J Agric Food Chem. 2013; 61: 6288-6292. Ref: https://goo.gl/GgK1Sf
- Nogueira JMF. Stir-bar sorptive extraction-15 years making sample preparation more environment friendly. TrAC. 2015; 71: 214-223. Ref: https://goo.gl/9j2Zyy
- Gallidabino M, Romolo FS, Bylenga K, Weyermann C. Development of a Novel Headspace Sorptive Extraction Method To Study the Aging of Volatile Compounds in Spent Handgun Cartridges. Anal Chem. 2014; 86: 4471-4478. Ref: https://goo.gl/wUDKVh
- Goudsmits E, Sharples GP, Birkett JW. Preliminary classification of characteristic organic gunshot residue compounds. Sci Justice. 2016; 56: 421-425. Ref: https://goo.gl/ajoJd3
- Goudsmits E, Sharples GP, Birkett JW. Recent trends in organic gunshot residue analysis. TrAC. 2015; 74: 46-57. Ref: https://goo.gl/pqPb13
- Meng H, Caddy B. Gunshot residue analysis - a review. J Forensic Sci. 1997; 42: 553-570. Ref: https://goo.gl/z6iQyG
- Dalby O, Butler D, Birkett JW. Analysis of gunshot residue and associated materials--a review. J Forensic Sci. 2010; 55: 924-943. Ref: https://goo.gl/s15FK1
- Taudte RV, Beavis A, Blanes L, Cole N, Doble P, et al. Detection of gunshot residues using mass spectrometry. Biomed Res Int. 2014; 2014: 1-16. Ref: https://goo.gl/gsN2CS
- Chang KH, Yew CH, Abdullah AF. Optimization of headspace solid-phase microextraction technique for extraction of volatile smokeless powder compounds in forensic applications, J Forensic Sci. 2014; 59: 1100-1108. Ref: https://goo.gl/nyJHSK
- Gassner AL, Weyermann C. LC-MS method development and comparison of sampling materials for the analysis of organic gunshot residues, Forensic Sci Int. 2016; 264: 47-55. Ref: https://goo.gl/mBxeut